Soil pH: Difference between revisions
m The LinkTitles extension automatically added links to existing pages (<a rel="nofollow" class="external free" href="https://github.com/bovender/LinkTitles">https://github.com/bovender/LinkTitles</a>). |
|||
| (20 intermediate revisions by 4 users not shown) | |||
| Line 1: | Line 1: | ||
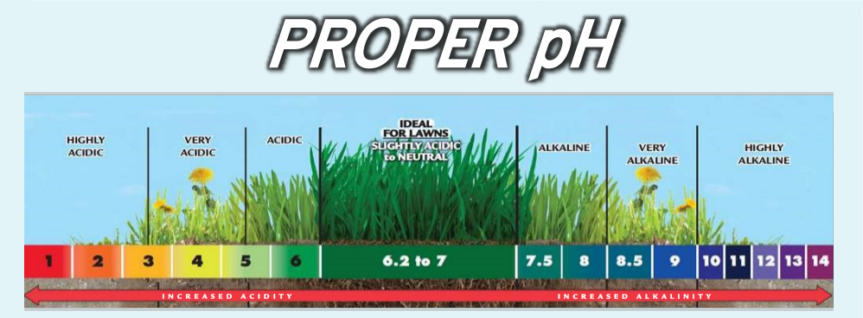
[[Soil]] pH is a measure of the acidity or basicity of a soil. Soil pH can be used as an important indicator to make | [[Soil]] pH is a measure of the acidity or basicity of a soil. Soil pH can be used as an important indicator to make both qualitative and quantitative analysis regarding soil characteristics. In soils, the pH is commonly measured as a slurry of soil mixed with water or a salt solution; the pH normally falls between 3 and 10, with 7 being neutral. Alkaline soils are basic soils with a pH above 7, while soils with a pH below 7 are acidic soils. Ultra-acidic soils which have a pH < 3.5 and very strongly alkaline soils which have a pH > 9 are rare, but can occur. The soil pH can affect many chemical processes and it specifically affects plant nutrient availability, influencing the chemical reactions they undergo. The best pH range for most plants is between 5.5 and 7.5, although many plants have adapted to survive at pH values outside this range [4]. | ||
| Line 33: | Line 33: | ||
|} | |} | ||
This classification of soil pH and classification is recognized and commonly used and recognized by many institutions but not limited to ESF [4]. | |||
== Methods of determining pH in soil == | == Methods of determining pH in soil == | ||
| Line 47: | Line 49: | ||
== Factors affecting soil pH == | == Factors affecting soil pH == | ||
====Sources of acidity==== | |||
* Rainfall: average rainfall has a pH of 5.6. When the | * Rainfall: average rainfall has a pH of 5.6. When the acid rain falls and flows into the soil it results in the leaching of basic cations from the soil. | ||
* | *Root respiration and [[decomposition]] of [[Organic Matter|organic matter]] by [[microorganisms]] releases carbon dioxide and increases carbonic acid concentration and subsequent leaching which acidifies the soil. | ||
* | * Fertilizer use, ammonium fertilizers react in the soil by nitrification to form nitrate, and in the process releases of Hydrogen ions; acidifying the soil. | ||
====Sources of alkalinity==== | |||
* Weathering of silicate, aluminosilicate, and carbonate materials will cause the soil basicity to rise. | * Weathering of silicate, aluminosilicate, and carbonate materials will cause the soil basicity to rise. | ||
| Line 62: | Line 62: | ||
* Addition of water containing dissolved bicarbonates, occurs when irrigating with high-bicarbonate waters. | * Addition of water containing dissolved bicarbonates, occurs when irrigating with high-bicarbonate waters. | ||
* Accumulation of basic elements such as carbonates and bicarbonates. occurring when insufficient water flows through the soils to leach the soluble salts. | |||
== Soil pH effects on plant growth == | == Soil pH effects on plant growth == | ||
====Acidic soils==== | |||
Plants grown in acidic soils can experience many stresses including aluminum, hydrogen, and/or manganese toxicity, as well as nutrient deficiencies of calcium and magnesium. | Plants grown in acidic soils can experience many stresses including aluminum, hydrogen, and/or manganese toxicity, as well as nutrient deficiencies of calcium and magnesium. | ||
Aluminum toxicity is the most widespread problem in acid soils. Aluminum is present in all soils to different degrees, dissolved aluminum is toxic to plants and aluminum is the most soluble at low pH, above a pH of 5 there is little aluminum soluble in most soils. Plants do not use aluminum as a nutrient and instead takes it up through osmosis through the [[plant roots]]. This uptake of aluminum has several effects on the growth of plants | Aluminum toxicity is the most widespread problem in acid soils. Aluminum is present in all soils to different degrees, dissolved aluminum is toxic to plants and aluminum is the most soluble at low pH, above a pH of 5 there is little aluminum soluble in most soils. Plants do not use aluminum as a nutrient and instead takes it up through osmosis through the [[plant roots]] [7]. This uptake of aluminum has several effects on the growth of plants due to high concentrations of aluminum inhibiting root growth. Thus causing lateral roots and root tips become thickened, and roots lack fine branching. | ||
====Alkaline soils==== | |||
Alkaline soils have low infiltration capacity so rain water stagnates on the soil easily and it is harder for plants to get established. Alkaline soils require irrigated water and good drainage. This soil is only used in agriculture for crops tolerant to surface | Alkaline soils have low infiltration capacity so rain water stagnates on the soil easily and it is harder for plants to get established [8]. Alkaline soils require irrigated water and good drainage. This soil is only used in [[agriculture]] for crops tolerant to surface water-logging such as grass and rice. The productivity of this soil is lower. | ||
== Water availability in relation to soil pH == | == Water availability in relation to soil pH == | ||
Strongly alkaline soils are sodic and have slow infiltration. | Strongly alkaline soils are sodic and have slow infiltration. Alkaline soils also have very poor available water capacity. Plant growth is restricted in wet conditions because aeration is poor when the soil is wet, and in dry conditions, water available to plants is rapidly depleted and the soils become hard. | ||
Strongly acidic soils have strong aggregation, good internal drainage and good water-holding characteristics. For many plant species aluminum toxicity severely limits root growth and moisture stress can occur when the soil is relatively moist. | Strongly acidic soils have strong aggregation, good internal drainage and good water-holding characteristics. For many plant species, aluminum toxicity severely limits root growth and moisture stress can occur when the soil is relatively moist [7]. | ||
== Changing soil pH == | == Changing soil pH == | ||
====Increasing pH of acidic soils==== | |||
Finely ground limestone is often applied to acid soils to increase soil pH called liming. The amount of liming needed depends on the mesh size used and the buffering capacity of the soil. The finer the mesh the faster it will react to soil acidity. The buffering capacity of a soil depends on its [[clay]] content and the amount of organic matter. Soils with a high buffering capacity will need a greater amount of liming to achieve an equivalent change in pH. | Finely ground limestone is often applied to acid soils to increase soil pH in a process called liming [6]. The amount of liming needed depends on the mesh size used and the buffering capacity of the soil. The finer the mesh, the faster it will react to soil acidity. The buffering capacity of a soil depends on its [[clay]] content and the amount of [[Organic Matter|organic matter]]. Soils with a high buffering capacity will need a greater amount of liming to achieve an equivalent change in pH [9]. | ||
====Decreasing pH of alkaline soils==== | |||
The pH level can be reduced by adding acidifying agents or acidic organic materials. Elemental sulfur has been used as it slowly oxidizes in soil to form sulfuric acid. Acidifying fertilizers such as ammonium sulfate, ammonium nitrate and urea can help reduce the pH of a soil because ammonium oxidizes to form nitric acid. Aluminum sulfate will also reduce the pH but the aluminum is also detrimental to plant growth. | The pH level can be reduced by adding acidifying agents or acidic organic materials. Elemental sulfur has been used as it slowly oxidizes in soil to form sulfuric acid. Acidifying fertilizers such as ammonium sulfate, ammonium nitrate and urea can help reduce the pH of a soil because ammonium oxidizes to form nitric acid. Aluminum sulfate will also reduce the pH, but the aluminum is also detrimental to plant growth [1]. | ||
== Gallery == | == Gallery == | ||
| Line 90: | Line 90: | ||
== References == | == References == | ||
1 | [1] Cox, L., and R. Koenig. (n.d.). Solutions to Soil Problems, II. High pH (alkaline soil) | ||
[2] nrcs142p2_053293.pdf. (n.d.). . | |||
[3] Queensland;, c=AU; o=The S. of. (n.d.). Soil pH | Soil [[properties]]. Text, corporateName=The State of Queensland; jurisdiction=Queensland. https://www.qld.gov.au/environment/land/management/soil/soil-properties/ph-levels. | |||
[4] Soil pH: What it Means. (n.d.). . | |||
https://www.esf.edu/pubprog/brochure/soilph/soilph.htm. | |||
[5] Magdoff, F., and R. Bartlett. 1985. Soil pH buffering revisited. Soil Sci Soc Am J. Soil Science Society of America Journal - SSSAJ 49. | |||
[6] Mclean, E. O. 1983. Soil pH and Lime Requirement. Pages 199–224 Methods of Soil Analysis. John Wiley & Sons, Ltd. | |||
[7] Robson, A. 2012. Soil Acidity and Plant Growth. Elsevier. | |||
[8] Sloan, J., and N. Basta. 1995a. Remediation of Acid Soils by Using Alkaline Biosolids. Journal of Environmental Quality - J ENVIRON QUAL 24. | |||
[9] Sloan, J. J., and N. T. Basta. 1995b. Remediation of Acid Soils by Using Alkaline Biosolids. Journal of Environmental Quality 24:1097–1103. | |||
[10] Thomas, G. W. 1996. Soil pH and Soil Acidity. Pages 475–490 Methods of Soil Analysis. John Wiley & Sons, Ltd. | |||
Latest revision as of 13:09, 10 May 2023
Soil pH is a measure of the acidity or basicity of a soil. Soil pH can be used as an important indicator to make both qualitative and quantitative analysis regarding soil characteristics. In soils, the pH is commonly measured as a slurry of soil mixed with water or a salt solution; the pH normally falls between 3 and 10, with 7 being neutral. Alkaline soils are basic soils with a pH above 7, while soils with a pH below 7 are acidic soils. Ultra-acidic soils which have a pH < 3.5 and very strongly alkaline soils which have a pH > 9 are rare, but can occur. The soil pH can affect many chemical processes and it specifically affects plant nutrient availability, influencing the chemical reactions they undergo. The best pH range for most plants is between 5.5 and 7.5, although many plants have adapted to survive at pH values outside this range [4].
Soil pH ranges and classification
| Denomination | pH range |
|---|---|
| Ultra acidic | < 3.5 |
| Extremely acidic | 3.5–4.4 |
| Very strongly acidic | 4.5–5.0 |
| Strongly acidic | 5.1–5.5 |
| Moderately acidic | 5.6–6.0 |
| Slightly acidic | 6.1–6.5 |
| Neutral | 6.6–7.3 |
| Slightly alkaline | 7.4–7.8 |
| Moderately alkaline | 7.9–8.4 |
| Strongly alkaline | 8.5–9.0 |
| Very strongly alkaline | > 9.0 |
This classification of soil pH and classification is recognized and commonly used and recognized by many institutions but not limited to ESF [4].
Methods of determining pH in soil
Observing a soil profile can reveal profile characteristics that can be possible indicators of acidic, alkaline or neutral soils.
- Poor incorporation of an organic surface layer with underlying mineral layer can indicate strongly acidic soils
- Columnar structure can be an indicator of sodic condition
- Presence of a caliche layer or a mineral deposit indicates the presence of calcium carbonates, which are present in alkaline conditions.
Observation of predominant flora. Calcifuge plants prefer acidic soils and include species from nearly all Ericaceae species, many birch, foxglove, gorse, and scots pine.
Use of an inexpensive pH testing kit where a small sample of soil can be mixed with an indicator solution which changes color according to the pH of the soil.
Use of a commercially available electronic pH meter in which a glass or solid-state electrode is inserted into moistened soil or a mixture of soil and water.
Factors affecting soil pH
Sources of acidity
- Rainfall: average rainfall has a pH of 5.6. When the acid rain falls and flows into the soil it results in the leaching of basic cations from the soil.
- Root respiration and decomposition of organic matter by microorganisms releases carbon dioxide and increases carbonic acid concentration and subsequent leaching which acidifies the soil.
- Fertilizer use, ammonium fertilizers react in the soil by nitrification to form nitrate, and in the process releases of Hydrogen ions; acidifying the soil.
Sources of alkalinity
- Weathering of silicate, aluminosilicate, and carbonate materials will cause the soil basicity to rise.
- Addition of water containing dissolved bicarbonates, occurs when irrigating with high-bicarbonate waters.
- Accumulation of basic elements such as carbonates and bicarbonates. occurring when insufficient water flows through the soils to leach the soluble salts.
Soil pH effects on plant growth
Acidic soils
Plants grown in acidic soils can experience many stresses including aluminum, hydrogen, and/or manganese toxicity, as well as nutrient deficiencies of calcium and magnesium. Aluminum toxicity is the most widespread problem in acid soils. Aluminum is present in all soils to different degrees, dissolved aluminum is toxic to plants and aluminum is the most soluble at low pH, above a pH of 5 there is little aluminum soluble in most soils. Plants do not use aluminum as a nutrient and instead takes it up through osmosis through the plant roots [7]. This uptake of aluminum has several effects on the growth of plants due to high concentrations of aluminum inhibiting root growth. Thus causing lateral roots and root tips become thickened, and roots lack fine branching.
Alkaline soils
Alkaline soils have low infiltration capacity so rain water stagnates on the soil easily and it is harder for plants to get established [8]. Alkaline soils require irrigated water and good drainage. This soil is only used in agriculture for crops tolerant to surface water-logging such as grass and rice. The productivity of this soil is lower.
Water availability in relation to soil pH
Strongly alkaline soils are sodic and have slow infiltration. Alkaline soils also have very poor available water capacity. Plant growth is restricted in wet conditions because aeration is poor when the soil is wet, and in dry conditions, water available to plants is rapidly depleted and the soils become hard.
Strongly acidic soils have strong aggregation, good internal drainage and good water-holding characteristics. For many plant species, aluminum toxicity severely limits root growth and moisture stress can occur when the soil is relatively moist [7].
Changing soil pH
Increasing pH of acidic soils
Finely ground limestone is often applied to acid soils to increase soil pH in a process called liming [6]. The amount of liming needed depends on the mesh size used and the buffering capacity of the soil. The finer the mesh, the faster it will react to soil acidity. The buffering capacity of a soil depends on its clay content and the amount of organic matter. Soils with a high buffering capacity will need a greater amount of liming to achieve an equivalent change in pH [9].
Decreasing pH of alkaline soils
The pH level can be reduced by adding acidifying agents or acidic organic materials. Elemental sulfur has been used as it slowly oxidizes in soil to form sulfuric acid. Acidifying fertilizers such as ammonium sulfate, ammonium nitrate and urea can help reduce the pH of a soil because ammonium oxidizes to form nitric acid. Aluminum sulfate will also reduce the pH, but the aluminum is also detrimental to plant growth [1].
Gallery
References
[1] Cox, L., and R. Koenig. (n.d.). Solutions to Soil Problems, II. High pH (alkaline soil)
[2] nrcs142p2_053293.pdf. (n.d.). .
[3] Queensland;, c=AU; o=The S. of. (n.d.). Soil pH | Soil properties. Text, corporateName=The State of Queensland; jurisdiction=Queensland. https://www.qld.gov.au/environment/land/management/soil/soil-properties/ph-levels.
[4] Soil pH: What it Means. (n.d.). . https://www.esf.edu/pubprog/brochure/soilph/soilph.htm.
[5] Magdoff, F., and R. Bartlett. 1985. Soil pH buffering revisited. Soil Sci Soc Am J. Soil Science Society of America Journal - SSSAJ 49.
[6] Mclean, E. O. 1983. Soil pH and Lime Requirement. Pages 199–224 Methods of Soil Analysis. John Wiley & Sons, Ltd.
[7] Robson, A. 2012. Soil Acidity and Plant Growth. Elsevier.
[8] Sloan, J., and N. Basta. 1995a. Remediation of Acid Soils by Using Alkaline Biosolids. Journal of Environmental Quality - J ENVIRON QUAL 24.
[9] Sloan, J. J., and N. T. Basta. 1995b. Remediation of Acid Soils by Using Alkaline Biosolids. Journal of Environmental Quality 24:1097–1103.
[10] Thomas, G. W. 1996. Soil pH and Soil Acidity. Pages 475–490 Methods of Soil Analysis. John Wiley & Sons, Ltd.