Decomposition
Introduction
Decomposition is the process in which large or complex molecules are broken down into simpler ones. This process is essential to a healthy ecosystem because it aids in the Nutrient Cycling of molecules such as phosphorus, nitrogen, water, carbon, and sulfur. In fact, soil organic matter, which includes plant or animal matter, holds three times as much carbon as either the atmosphere or living vegetation [1]. This is important because it is carbon and nitrogen that often limits the productivity of an ecosystem. Factors that affect decomposition rate are temperature, water content, climate, soil type, and substrate quality [2].

While macroorganisms such as earthworms, flies, insects, and snails are involved in the early process of decomposition, it is often the work of enzymes, bacteria, and fungi that aid in the cycling of nutrients back into the soil [3].
Quantification
The quantification of litter breakdown has only been established in recent years, and describes the relationship between existing litter, annual production, and time. The decomposition of organic matter can be described by the following equations:
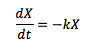
k=rate of breakdown
X=litter on ground
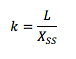
L=annual production
Xss=base level of litter
Different climates and regions will have different rates of decomposition based on the ratio between the annual production and the base level of litter. For example, rates within evergreen forests vary widely. In tropical forests the rate is 4, in eastern pine it is .25, and in alpine taiga it is .02. This is because the temperature and moisture of the region heavily impacts the value of k. Decomposition, in general, is very difficult to measure on a wide scale due to the heterogeneity of soil and litter. One way to measure decomposition is burying mesh leaf litter bags, which help to isolate an area of interest and test on a small scale. Large scale and long term experiments are much more difficult. Decomposition will never result in zero litter remaining. This is because the remaining matter is highly recalcitrant, meaning it has a high resistance to breakdown. This is because the remaining compounds are lignins, fats, and cellulose. This may also include some resistant polymers, by-products of microbial decomposition [3].
Molecular Breakdown
Breakdown of Detritus
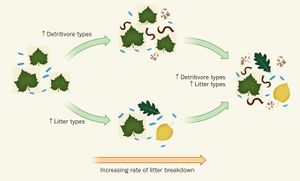
In the early stages of decomposition, detritivores and other organisms will begin to consume the dead organic matter. Detritivores eat detritus, the name given to disintegrated organic materials. These macroorganisms break apart large material such as plant and animal residue, tissue of soil organisms, and any substances produced by soil organisms. By breaking down these large particles, they increase the surface area available for bacteria and fungi.
While detritivores aid in the initial stages of decomposition, it is the work of fungi and bacteria that metabolize organic matter and break it down into inorganic compounds. The fungi and bacteria that thrive on dead matter are called saprophytes [3]. Saprophytes can secrete chemicals that digest the molecules and result in the mineralization of the compounds.
Mineralization
Mineralization is the process by which organic compounds are broken down into water-soluble inorganic compounds as the result of microbial activity [4]. These compounds are broken down by microorganisms like fungi and bacteria, which secrete chemicals that aid in decomposition. These chemicals include enzymes, which can decompose plant litter that contain high amounts of cellulose and lignin [2]. There are various forms of enzymes, which aid in the breakdown of different types of compounds. For example, oxidative enzymes are best at decomposing complex substrates like lignin, while hydrolytic enzymes breakdown simpler compounds such as starches and sugars. The process of mineralization is essential to nutrient cycling because it allows insoluble organic compounds to become water-soluble and available to plants. Mineralization is one of the main processes, which occur in carbon and nitrogen cycling. These cycles are essential to the livelihood of an ecosystem.
Different molecules within soil organic matter breakdown at different speeds depending on their molecular structure. From fastest to slowest, the breakdown is as follows:
1. Sugars, starches, and simple proteins
2. Proteins
3. Hemicelluloses
4. Cellulose
5. Lignins and fats
When the mineralization of a compound is complete, it becomes bioavailable for plants to use, thus recycling the nutrient from the dead plant or animal back into the system. This partially digested, nutrient-rich, and bioavailable soil is called humus.
Humification
Humification is the process by which organic matter, which has already been mineralized, is further broken down through the processes of weathering, freeze-thaw cycle, and erosion. This physical decomposition allows it to be more available to plants for use. Humus is mostly found in the topsoil layer, and as the soil is undergoing physical weathering, the water-soluble minerals leech into the surrounding soil water by the force of gravity. As the minerals travel down into the soil, plant roots can uptake this mineral rich water. Humus also helps to retain soil moisture and keeps soil aerated [3].
References
[1] Schmidt M.W.I., Torn M.S., Abiven S., Dittmar T., Guggenberger G., Janssens I.A., Kleber M., Kögel-Knabner I., Lehmann J., Manning D.A.C., Nannipieri P., Rasse D.P., Weiner S., and Trumbore S.E. 2011. Persistence of soil organic matter as an ecosystem property. Nature 478 (7367): 49.
[2] “Decomposition.” Soil Biology, biology.soilweb.ca/decomposition/.
[3] Terry, Watkins. “Decomposition.” Organic, Process, Soil, and Humus - JRank Articles, science.jrank.org/pages/1967/Decomposition.html.
[4] Olson, J. S. 1963. Energy storage and the balance of producers and decomposers in ecological systems. Ecology 44:322-331.
[5] Ecology: Diversity in the afterlife, N&V, Nature 509, 173–174 (08 May 2014) doi:10.1038/509173a
[6] “What Is Soil?” All About Soil | Soils 4 Kids, Soil Science Society of America, www.soils4kids.org/about.