Soil Sampling Methods: Difference between revisions
m The LinkTitles extension automatically added links to existing pages (<a rel="nofollow" class="external free" href="https://github.com/bovender/LinkTitles">https://github.com/bovender/LinkTitles</a>). |
|||
| (75 intermediate revisions by 3 users not shown) | |||
| Line 1: | Line 1: | ||
[[Soil]] sampling gives is useful for determining the structure and function of the [[soil|soil]] community. Study subjects include type of [[organisms|organisms]] living in the soil, the [[plant roots|plant roots]] structure, leaf litter break down and [[decomposition]]. There are different methods of soil sampling, for measurement of different target [[organisms]] and variables. | |||
[[File:baermann.png|thumb|Baermann Funnel, Bhat and Rao 2020]] | |||
== | == Nematode Sampling == | ||
Baermann funnels are useful for measurement of microfauna and [[microorganisms|microorganism]] abundance and richness in a soil sample. A funnel with a small mesh or muslin lining on the inside is attached via tube to the bottom of the funnel, which is clamped shut at the bottom. When the soil is placed in the funnel, it is then filled with water. Organisms like [[nematodes|nematodes]] will move down through the soil and water, through the mesh and into the bottom of the tube. After 24 to 48 hours, the clamp is opened, allowing the organisms to drain into a petri dish. Samples can then be identified via microscopy. | |||
== Ground Level Arthropod Sampling == | |||
[[File:Berlese_tullgren.png|thumb|Berlese-Tullgren funnel for micro-arthropods extraction. It contains a sample container with wire mesh, a plastic funnel over which the sample container is placed and a collecting vessel below the funnel which contains a liquid preservative. Sapkota et al 2012]] | |||
Tullgren funnels are used to assess species richness in leaf litter [[arthropods]]. Leaf litter samples are typically placed in Tullgren funnels to sample for soil micro- and [[mesofauna]]. The Tullgren apparatus consists of a funnel, a light source, a screen, and a receptacle into which the [[animals]] fall. The sample is placed in a funnel that is lined with a screen with the light source above; the extraction process works by desiccating the soil from above via heat lamp, forcing the fauna downward through the funnel into the sampling liquid below. | |||
Leaf litter packs can also consist of a mesh bag, usually with 1/4 inch openings filled with a mixture of palatable organic material like fresh leaf litter and lettuce. The size in the mesh is varied based on the size of target [[organisms|organisms]]. The mesh is large enough so target organisms can crawl into the bag, and small enough so they generally can not leave. Leaf packs should be placed on a low spot on the ground in order to maximize capture: this is where soil is most fertile, and where moisture content is highest. A small hole is dug in the ground, into which the bag is placed and then loosely covered with [[soil|soil]] and surrounding leaves. Marking the spot with a bag is generally recommended so that the bag can be retrieved later. The leaf pack is generally left for a 1-3 days to allow organisms to get into the bag before removal. Next, you dump the leaves out of the bag onto a plate and start looking for organisms like [[slugs]], worms, centipedes, [[mites]], and smaller organisms. You can put them on a petri dish and observe the [[soil organisms|soil organisms]] closer under the microscope to allow you to properly identify them. | |||
[[File:Leaf litter pack.jpg|left thumb|Leaf litter pack ready to be placed]] | |||
== | == Root Sampling == | ||
[[File:Rhizotron.jpg|thumb| Underground rhizotron]] | |||
Rhizotrons come in various sizes, which allow for study at multiple scales. The largest example of a rhizotron is a tunnel built underground with windows placed all around the tunnel, allowing for non-destructive study of the roots and rhizosphere. Smaller rhizotrons can be built with a sturdy, clear material for study of individual plants and smaller systems. The roots grow up to the windows which allows us to see the way the roots grow. We can see the roots along with the [[root hairs]] that grow off the roots. Using this method we can see how thick roots are, how deep they go and how wide spread the roots grow for different plants in different [[soil|soil]] types. Using a rhizotron is very useful in a forest for research as scientists can measure and observe the interactions that occur on and [[rhizosphere]]. From the roots holding down the tree or other plants, to the interactions with the micro organisms living next to the root hairs. Scientists can learn a lot for using a rhizotron in a way that they do not have to dig up all the roots to see how they look and work. | |||
== References == | |||
[2] Bhat A.I., Rao G.P. (2020) Transmission of Viruses Through [[Nematodes]]. In: Characterization of Plant Viruses. Springer Protocols Handbooks. Humana, New York, NY. https://doi.org/10.1007/978-1-0716-0334-5_19 | |||
---- | [3] Berlese, Antonio (1905). Apparecchio per raccogliere presto ed in gran numero piccoli Artropodi (Apparatus for gathering early and in large numbers small arthropods) (in Italian). pp. 85–90. | ||
[4] Tullgren, A (26 August 2009). "Ein sehr einfacher Ausleseapparat für terricole Tierformen" [A very simple readout device for terricole animal forms]. Zeitschrift für Angewandte Entomologie (in German). | |||
[5] Benfield EF (2006) Decomposition of leaf material. In: Hauer FR, Lamberti GA (eds) Methods in stream [[ecology]], 2nd edn. Academic Press, Burlington, pp 12 | |||
[6] Sapkota, T.B., Mazzoncini, M., Bàrberi, P. et al. Fifteen years of no till increase soil [[Organic Matter|organic matter]], microbial biomass and [[arthropod]] diversity in cover crop-based arable cropping systems. Agron. Sustain. Dev. 32, 853–863 (2012). https://doi.org/10.1007/s13593-011-0079-0 | |||
Bug Hunter, bughunter.tamu.edu/collection/collectionequipment/berlese-funnel/. | |||
“What Is a Rhizotron?” Effects of [[Emerald Ash Borer]] on Forest Ecosystems - Emerald Ash Borer - Forest Disturbance Processes - Northern Research Station - USDA Forest Service, www.nrs.fs.fed.us/research/facilities/rhizotron/. | |||
“Proper Soil Sampling Techniques.” Volusia County, www.volusia.org/services/community-services/extension/agriculture/proper-soil-sampling-techniques.stml. | |||
“Leaf Pack Study (Department of Ecosystem Science and Management).” Department of Ecosystem Science and Management (Penn State University), ecosystems.psu.edu/youth/sftrc/lesson-plans/water/6-8/leafpack. | |||
Animal [[Diversity]] Web, animaldiversity.org/accounts/Arthropoda/classification/. | |||
Mohamed, Awaz, et al. “An Evaluation of Inexpensive Methods for Root Image Acquisition When Using Rhizotrons.” Plant Methods, BioMed Central, 7 Mar. 2017, plantmethods.biomedcentral.com/articles/10.1186/s13007-017-0160-z. | |||
Latest revision as of 13:30, 31 March 2023
Soil sampling gives is useful for determining the structure and function of the soil community. Study subjects include type of organisms living in the soil, the plant roots structure, leaf litter break down and decomposition. There are different methods of soil sampling, for measurement of different target organisms and variables.

Nematode Sampling
Baermann funnels are useful for measurement of microfauna and microorganism abundance and richness in a soil sample. A funnel with a small mesh or muslin lining on the inside is attached via tube to the bottom of the funnel, which is clamped shut at the bottom. When the soil is placed in the funnel, it is then filled with water. Organisms like nematodes will move down through the soil and water, through the mesh and into the bottom of the tube. After 24 to 48 hours, the clamp is opened, allowing the organisms to drain into a petri dish. Samples can then be identified via microscopy.
Ground Level Arthropod Sampling
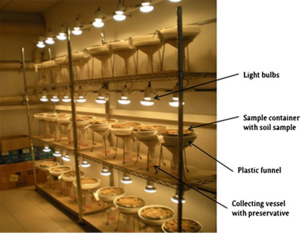
Tullgren funnels are used to assess species richness in leaf litter arthropods. Leaf litter samples are typically placed in Tullgren funnels to sample for soil micro- and mesofauna. The Tullgren apparatus consists of a funnel, a light source, a screen, and a receptacle into which the animals fall. The sample is placed in a funnel that is lined with a screen with the light source above; the extraction process works by desiccating the soil from above via heat lamp, forcing the fauna downward through the funnel into the sampling liquid below.
Leaf litter packs can also consist of a mesh bag, usually with 1/4 inch openings filled with a mixture of palatable organic material like fresh leaf litter and lettuce. The size in the mesh is varied based on the size of target organisms. The mesh is large enough so target organisms can crawl into the bag, and small enough so they generally can not leave. Leaf packs should be placed on a low spot on the ground in order to maximize capture: this is where soil is most fertile, and where moisture content is highest. A small hole is dug in the ground, into which the bag is placed and then loosely covered with soil and surrounding leaves. Marking the spot with a bag is generally recommended so that the bag can be retrieved later. The leaf pack is generally left for a 1-3 days to allow organisms to get into the bag before removal. Next, you dump the leaves out of the bag onto a plate and start looking for organisms like slugs, worms, centipedes, mites, and smaller organisms. You can put them on a petri dish and observe the soil organisms closer under the microscope to allow you to properly identify them.
Root Sampling

Rhizotrons come in various sizes, which allow for study at multiple scales. The largest example of a rhizotron is a tunnel built underground with windows placed all around the tunnel, allowing for non-destructive study of the roots and rhizosphere. Smaller rhizotrons can be built with a sturdy, clear material for study of individual plants and smaller systems. The roots grow up to the windows which allows us to see the way the roots grow. We can see the roots along with the root hairs that grow off the roots. Using this method we can see how thick roots are, how deep they go and how wide spread the roots grow for different plants in different soil types. Using a rhizotron is very useful in a forest for research as scientists can measure and observe the interactions that occur on and rhizosphere. From the roots holding down the tree or other plants, to the interactions with the micro organisms living next to the root hairs. Scientists can learn a lot for using a rhizotron in a way that they do not have to dig up all the roots to see how they look and work.
References
[2] Bhat A.I., Rao G.P. (2020) Transmission of Viruses Through Nematodes. In: Characterization of Plant Viruses. Springer Protocols Handbooks. Humana, New York, NY. https://doi.org/10.1007/978-1-0716-0334-5_19
[3] Berlese, Antonio (1905). Apparecchio per raccogliere presto ed in gran numero piccoli Artropodi (Apparatus for gathering early and in large numbers small arthropods) (in Italian). pp. 85–90.
[4] Tullgren, A (26 August 2009). "Ein sehr einfacher Ausleseapparat für terricole Tierformen" [A very simple readout device for terricole animal forms]. Zeitschrift für Angewandte Entomologie (in German).
[5] Benfield EF (2006) Decomposition of leaf material. In: Hauer FR, Lamberti GA (eds) Methods in stream ecology, 2nd edn. Academic Press, Burlington, pp 12
[6] Sapkota, T.B., Mazzoncini, M., Bàrberi, P. et al. Fifteen years of no till increase soil organic matter, microbial biomass and arthropod diversity in cover crop-based arable cropping systems. Agron. Sustain. Dev. 32, 853–863 (2012). https://doi.org/10.1007/s13593-011-0079-0
Bug Hunter, bughunter.tamu.edu/collection/collectionequipment/berlese-funnel/.
“What Is a Rhizotron?” Effects of Emerald Ash Borer on Forest Ecosystems - Emerald Ash Borer - Forest Disturbance Processes - Northern Research Station - USDA Forest Service, www.nrs.fs.fed.us/research/facilities/rhizotron/.
“Proper Soil Sampling Techniques.” Volusia County, www.volusia.org/services/community-services/extension/agriculture/proper-soil-sampling-techniques.stml.
“Leaf Pack Study (Department of Ecosystem Science and Management).” Department of Ecosystem Science and Management (Penn State University), ecosystems.psu.edu/youth/sftrc/lesson-plans/water/6-8/leafpack.
Animal Diversity Web, animaldiversity.org/accounts/Arthropoda/classification/.
Mohamed, Awaz, et al. “An Evaluation of Inexpensive Methods for Root Image Acquisition When Using Rhizotrons.” Plant Methods, BioMed Central, 7 Mar. 2017, plantmethods.biomedcentral.com/articles/10.1186/s13007-017-0160-z.
