Root hairs
Overview
Root hairs provide an interface between larger plant roots and the soil. Aside from the larger more commonly known system, root hairs are smaller cylindrical extensions of larger roots that are important for nutrient acquisition, microbial interactions below-ground, and for plant anchorage in the soil. Root hairs are substantially beneficial to the health of a plant and its root systems because it effectively increases the surface area and diameter of the roots [1]. Root hairs can be 10μm in diameter or up to 1mm in diameter. The study of root hairs is important for ecology, cell biology, and plant physiology because of their unique cell makeup and cell growth. Root hairs are often players in the formation of root nodules on legume plants; these facilitate symbiotic mycorrhizal interactions. Root hairs are usually visible to the naked eye but are better seen with a compound microscope or electron microscope.
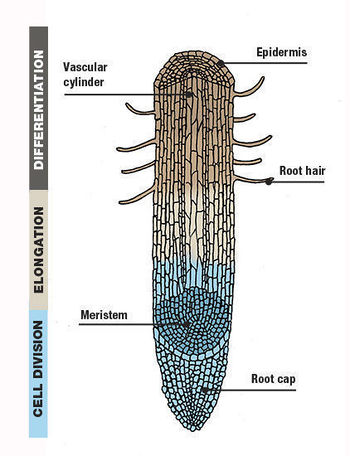
Growth and Development
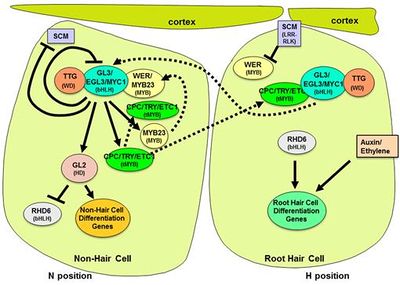
Root hairs are excellent specimens to study in the field of cell biology. Root hairs are tip growing extensions from specialized root epidermal cell (trichoblasts); in most angiosperms root hairs develop on epidermal cells in the differentiation zones of younger roots [2]. In most eudicots, the first sign of root hair development is a bulge on the outer radial wall (periclinal wall) of the epidermal cell [2]. Cells of the plant epidermis in the differentiation zone can either become a root hair or non root hair cell. After the basic understanding that the root epidermis consists of root hair cells and non root hair cells, it is important to understand that there are three different types patterns of differentiation that have been seen to occur in the development of root hairs [3].
Type 1:
Root hairs can emerge from any kind of cell and can be considered a random type.
Type 2:
Root hairs develop from a specific population of root epidermal cells composed of vertically alternating short and long cells; root hairs will emerge from the short cells.
Type 3:
Root hairs grow from cells that are localized between two cortical cells (cell in the cortex) in which non hair cells contact only one cortical cell. This makes for adequate spacing between root hair growths.
Auxin, a signaling hormone used for regulating the growth of almost all plant development was previously studied mainly in the aboveground development of plant leaves and shoots. Recently, genetic and physiological evidence has shown that auxin is also a key player in the growth of both lateral roots and root hairs below-ground [4].
Root Hair Surface Area
Recent developments in technology used in studies to show the below-ground biomass of the root systems of plants has aided in studying the mass and surface area of tiny root hairs that were previously uncountable or immeasurable. In grassland plants such as Secale Cereale (Winter Rye), preliminary studies done on the counting of root hairs shows that root hairs alone can amount to up to 11, 483, 271 compared to around 2 million larger roots [5]. It was shown in this study that root hairs from just one rye plant can account for 4,322 square feet below ground. This remarkable discovery not only shows how much root hairs contribute to below-ground biomass, but points to root hair's importance in nutrient acquisition and water uptake.
Nutrient Acquisition
Previously mentioned, root hairs play a significant role in nutrient acquisition because of their small size and role in increasing the surface area of the below-ground root system. Root hairs are especially important for the uptake of phosphorus (P) and potassium (K) for the health and growth of the shoots above ground [3]. Not only do root hairs increase nutrient acquisition through their extension of root surface area into soil spaces that could not otherwise be reached, root hairs also increase the preferential expression of enzymes involved in the mobilization of and uptake of multiple nutrients that are vital to plant growth [6]. These extensions of the root systems contribute to increased surface area as discussed above which improves soil contact, and can account for up to 80% of P intake [7]. Improving P uptake in particularly P deficient soil lays in the hands of root hairs. Certain root morphological and architectural adaptations can increase total soil exploration by altering the size, angle, and length of the root hairs. It has also been shown that even in P deficient environments, root hair growth increases in order to locate sparse sources of P in the soil. This has been seen with other vital nutrients as well such as Pi, K, N, and C [7].
Water Uptake and Hydraulics
Root hairs also play significant roles in water uptake, similar to nutrient acquisition. Roots consist of many zones. Lateral roots and root caps sometimes exist in regions that have low water permeability due to immature water conduit and root suberization [8]. It has been shown that the root hair zone tends to be the most permeable zone which suggests the root hair's contributions to a plant's water uptake ability. Root water uptake is increased not only because of increased surface area of roots in small crevices of soil, but also because these small root hairs increase water potential which physically makes water uptake easier and more possible [8].
Sampling methods/limitations
Sequential Soil Coring
Root cores can be used to obtain information about root hair length and mass, number of root tips, mycorrhizal biomass, and distribution of nutrient contents. Iron cylinders are driven into penetrable soils at a desired sampling depth. This is hard to use past 1m because the presence of stones can prohibit the use of the coring tools to greater depths [11]. The limitations of this method are that it can be time consuming, which limits the amounts of samples that can be obtained during the growing period. [11]
Ingrowth coring
This method is comparatively quicker and less laborious compared to the sequential soil coring method [11]. A specified volume of soil is removed from the sampling area, and this volume of soil is replaced with root free soil surrounded by a mesh bag. These bags are filled with root free sieved mineral soil from the study area; these mesh bags are then resampled after a defined time period. The varying growth of roots in this microsite can then be studied [11]. Major limitations of this method are that the soil mesh bag provides a high nutrient and low competition environment which recolonizes at different rates compared to other parts of the soil [11].
Minirhizotron
This is a nondestructive method that can be useful for measuring the same root over longer time periods, which the ingrowth coring method does not offer [11]. Minirhizotrons are glass tubes that are installed into the soil, and root intersections along the tube can be viewed with a small camera. These can be used to obtain quantitative data on root length and production, root longevity, root density and root diameter [11]. Similar to the sequential soil coring method discussed previously, limitations of minirhizotrons include difficulty implementing them into stony soils.
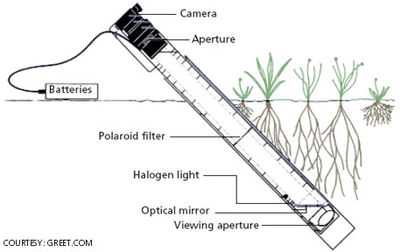
Image Analysis
The program Image J can be used to measure root hair size and density. Below are some steps that can be taken to do so [10].
- Obtain two petri dishes - one will contain DI water, the other will contain Toluidine blue dye.
- Using forceps, carefully submerge the root hair in the blue dye, after, carefully move the root hair to the DI water to rinse the excess blue dye off.
- Using a image capturing microscope, adjust scale and capturing speed appropriately and lights on microscope to capture an image of the root hair that can be used in ImageJ
- Upload image to ImageJ
- Open the calibration image. Use the line tool to draw a line of known distance next to the calibration image (ruler). Click "Analyze" "Set Scale" and set the known distance to the distance measured and set to global.
- Import your images using "File" "Import" "Image Sequence"
- When image is uploaded, use "Analyze" "Set Measurements" to find "Display label"
- To measure the length of the root hairs, select the line tool to trace and measure the root hair length in the images. Hit "M" to record in the results window. Record these results in a seprate spreadsheet.
- To measure the root hair density, use the rectangle tool on preferred image to select a representative area of the root. Hit "M" to record the area.
- Visually count the number of root hairs in the selected area. Record this in spreadsheet
- When done, divide the recorded root hair count divided by the recorded area to obtain the number of root hairs per mm^2
References
[1] Grierson, C., E. Nielsen, T. Ketelaarc, and J. Schiefelbein. 2014. Root Hairs. The Arabidopsis Book / American Society of Plant Biologists 12.
[2] Datta, S., C. M. Kim, M. Pernas, N. D. Pires, H. Proust, T. Tam, P. Vijayakumar, and L. Dolan. 2011. Root hairs: development, growth and evolution at the plant-soil interface. Plant and Soil 346:1–14.
[3] Crespi, M. 2012. Root Genomics and Soil Interactions. John Wiley & Sons, Incorporated, Somerset, UNITED STATES.
[4] Santelia, D., V. Vincenzetti, E. Azzarello, L. Bovet, Y. Fukao, P. Düchtig, S. Mancuso, E. Martinoia, and M. Geisler. 2005. MDR-like ABC transporter AtPGP4 is involved in auxin-mediated lateral root and root hair development. FEBS Letters 579:5399–5406.
[5] Dittmer, Howard J. A Quantitative Study of the Roots and Root Hairs of a Winter Rye Plant (Secale Cereale). American Journal of Botany, vol. 24, no. 7, 1937, pp. 417–420. JSTOR, www.jstor.org/stable/2436424.
[6] Salazar-Henao, J. E., and W. Schmidt. 2016. An Inventory of Nutrient-Responsive Genes in Arabidopsis Root Hairs. Frontiers in Plant Science 7.
[7] Haling, R. E., L. K. Brown, A. G. Bengough, I. M. Young, P. D. Hallett, P. J. White, and T. S. George. 2013. Root hairs improve root penetration, root–soil contact, and phosphorus acquisition in soils of different strength. Journal of Experimental Botany 64:3711–3721.
[8] Segal, E., T. Kushnir, Y. Mualem, and U. Shani. 2008. Water uptake and hydraulics of the root hair rhizosphere. Vadose Zone Journal 7:1027–1034.
[9] Caring for Plant Roots: What You Need to Know. 2017, March 18. . https://www.finegardening.com/article/caring-for-plant-roots-what-you-need-to-know.
[10] Penn State College of Agricultural Sciences. 2020. Imaging and Analyzing Root Hair Length and Density. https://plantscience.psu.edu/research/labs/roots/methods/methods-info/root-hairs/root-hair-imaging-protocol/imaging-and-analyzing-root-hair-length-and-density
[11] Majdi, Hooshang. Root Sampling Methods - Applications and Limitations of the Minirhizotron Technique. Plant and soil, vol. 185, no. 2, 1997, pp. 255-288. JSTOR, https://www-jstor-org.gate.lib.buffalo.edu/stable/42947826?sid=primo&seq=3#metadata_info_tab_contents