Mycorrhizae
Mycorrhizae is a symbiotic association between plant roots and fungi. The formation of a mycorrhizal symbiosis is considered a widespread strategy of plants to obtain advantages under selection pressure in natural ecosystems. [2] The fungi in the soil which commonly form mycorrhizal relationships with plants are mostly unique. The relationships between the fungus and the plant are mutual beneficial: the fungus receives necessary carbohydrates from the host plant, and the mycorrhizae absorbs nutrients from the soil which are passed along to the plant in return. Mycorrhizae is one of the important set of survival mechanisms for plants living in challenging locations. [3] It also play a key role in terrestrial ecosystems to regulate nutrient and carbon cycles, and influence soil structure and ecosystem multifunctionality. [4] There are mainly two types of mycorrhizae based on morphology and physiology characteristics: Ectomycorrhizae and Endomycorrhizae.
Ectomycorrhizae

A: with the basidiomycetous truffle Hysterangium;
B: mycorrhizal fungus Rhizopogon vinicolor;
C: mycorrhizal fungus Poria terrestris;
D: mycorrhizal fungus Lactarius sanguifluus;
E: Sitka spruce;
F: Monterey pine. [5]
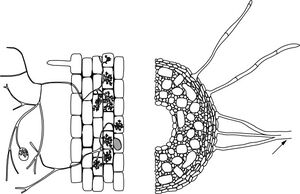
Ectomycorrhizae (ECM) are characterized by the presence of a fungal mantle that envelops host roots and a Hartig net that surrounds root epidermal or cortical cells. It provides a large surface area for resource exchange. Hormonal interactions between plant and fungus lead to dramatically altered root architecture, such as the suppression of root hairs. The external component of Ectomycorrhizae associations consists of hyphae with cross walls that partition cellular components. These hyphae sometimes coalesce into rhizomorphs that attach the mycelium to serve in water uptake.[6]
Ectomycorrhizae associations involve the most diverse category of myocrrhizae: more than 5,000 species of fungi (mainly Basidiomycetes) with a limited number of Ascomycetes and Zygomycetes. [7] Major groups of plants for ECM include most herbs, grasses and many trees, hornworts, and liverworts. There are approximately 200,000 number of plant species hosing Ectomycorrhize fungi. [8] The majority of ECM synthesizing fungi belong to the classes Basidiomycetes and Ascomycetes that form fruiting bodies like mushrooms, puffballs, coral fungi, toadstools, truffles, etc. [9] Ectomycorrhizae are more common in the temperate zones of the world comparing to the tropics. In the northern temperate regions, plants such as pine (Pinus), spruce (Picea), fir (Abies), poplar (Populus), willow (Salix), beech (Fagus), birch (Betula) and oak (Quercus) typify the Ectomycorrhizae association. In the southern hemisphere, Eucalyptus and Northofagus (Southern Beech) are important genera. In total, 140 genera in 43 plant families have been identified as forming Ectomycorrhizae.[10]
Endomycorrhizae
The fungi form structures within the cortical cells and grow intercellularly. The membranes of the fungus and the plant are in direct contact with each other. [11] Endomycorrhizaes include arbuscular mycorrhizae (vesicular-arbuscular mycorrhiae), ericoid mycorrhizae and orchid mycorrhizae. [12]
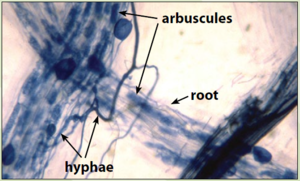
Arbuscular Mycorrhizae
Arbuscular mycorrhiza (AM) is a symbiosis between plants and members of an ancient phylum of fungi, the Glomeromycota. It improves the supply of water and nutrients, such as phosphate and nitrogen, to the host plant. Up to 20% of plant-fixed carbon is transferred to the fungus. AM development is accompanied by an exchange of signalling molecules between the symbionts. [14] AM form vesicles, arbuscules, and hyphae in roots, and also spores and hyphae in the rhizosphere. Formation of hyphal network by the AM with plant roots significantly enhances the access of roots to a large soil surface area, causing improvement in plant growth. AM improve plant nutrition by increasing the availability as well as translocation of various nutrients; improve the the quality of soil by influencing its structure and texture, and hence plant health. [15]
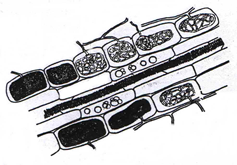
Ericoid Mycorrhizae
Ericoid mycorrhizae (ERM) are a restricted group of fungi associated with a restricted diversity of plant species in the Ericaceae, Epacridaceae, and Empetraceae. Hymenoscyphus (Pezizella) ericae was the first fungal species identified as an ericaceous endosymbiont. [17]. ERM fungi are versatile fungi because, besides promoting growth and health of ericaceous plants as endomycorrhizal symbionts, they are also reported as endophytes in the roots of other plant species. [18] Colonisation by ERM is restricted to expanded epidermal cells (i.e. mature cells),therefore the apical region of the hair root remains uncolonised until the cells differentiate and mature. [19]
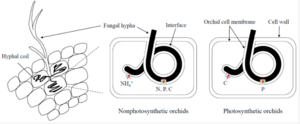
Orchid Mycorrhizae
Orchid mycorrhizae is an endomycorrhizal association with an extensive intracellular mycelium, occurring in the roots of the members of the Orchidaceae. Orchid mycorrhizae supports host plant with nutrients, especially sugars and water. The transport of carbon compounds from fungus to host plant may be realized through the plasmatic membrane of living pelotons (in trophocytic phase), or by phagocytosis of collapsed fungal endophyte (in phagocytic phase). [21] The infection of an orchid seed by fungi occurs after the embryo takes up water and swells, rupturing the seed coat. The embryo emerges and produces a few root hairs, which hyphae rapidly colonise. When hyphae penetrate a cell of the embryo, the plasma membrane of the orchid cell invaginates, and the hypha becomes surrounded by a thin layer of cytoplasm. An orchid embryo consists of a few hundred cells and the fungi spread quickly from cell to cell. [22]
References
- ↑ Marschner, P. (2012). Rhizosphere Biology. In P. Marschner (Eds.), Marschner's Mineral Nutrition of Higher Plants (Thrid Edition). Academic Press. pp, 369-388.
- ↑ Schönbeck,F., Grunewaldt-Stöcker, G. & von Alten, H. (1994). Mycorrhizae. In C. L. Campbell, D. M. (Eds.), Epidemiology and Management of Root Diseases Benson. Springer, Dordrecht.
- ↑ O’Callaghanm A.M. Mycorrhizae Fact Sheet. https://www2.nau.edu/~gaud/bio300/mycorrhizae.htm
- ↑ van der Heiden, M. G.A., Martin, F.M., Selosse, M., & Sanders, I.R. (2015). Mycorrhizal ecology and evolution: the past, the present, and the future. New Phytologist, 205(4), 1406-1423.
- ↑ MMoore, D., Ectomycorrhizas. http://www.davidmoore.org.uk/assets/mostly_mycology/diane_howarth/ectomycorrhizas.htm
- ↑ Nancy, C., & Gehring, C. A. (2007). Mycorrhizas: Symbiotic Mediators of Rhizosphere and Ecosystem Processes. In Z.G.Cardon and J. L. Whitebeck (Eds.). The Rhizosphere. Academic Press.
- ↑ Futai, K., Taniguchi, T., Kataoka, R. (2008). Ectomycorrhizae and Their Importance in Forest Ecosystems. In: Siddiqui, Z.A., Akhtar, M.S., Futai, K. (Eds). Mycorrhizae: Sustainable Agriculture and Forestry. Springer, Dordrecht.
- ↑ van der Heiden, M. G.A., Martin, F.M., Selosse, M., & Sanders, I.R. (2015). Mycorrhizal ecology and evolution: the past, the present, and the future. New Phytologist, 205(4), 1406-1423.
- ↑ Charya, L. S., & Garg, S. (2019). Advances in methods and practices of ectomycorrhizal research. In S. N. Meena and M. M. Nalk (Eds.). Advances in Biological Science Research. Academic Press.
- ↑ Moore, D., Ectomycorrhizas. http://www.davidmoore.org.uk/assets/mostly_mycology/diane_howarth/ectomycorrhizas.htm
- ↑ Marschner, P. (2012). Rhizosphere Biology. In P. Marschner (Eds.), Marschner's Mineral Nutrition of Higher Plants (Thrid Edition). Academic Press. pp, 369-388.
- ↑ Reddy, C. A. & Saravananm, R. S. (2013). Polymicrobial Multi-functional Approach for Enhancement of Crop Productivity, Advances in Applied Microbiology. 82, 53-113.
- ↑ Smith, S., Manjarrez, M., & Smith, A. Fact Sheets - Arbuscular Mycorrhizas - SA, https://soilquality.org.au/factsheets/arbuscular-mycorrhizas-s-a.
- ↑ Parniske, M. (2008). Arbuscular mycorrhiza: the mother of plant root endosymbioses, Nature Reviews Microbiology, 6, 763-775.
- ↑ Zou, Y. N., Srivastava, A. K., & Wu, Q. S.(2016). Glomalin: a potential soil conditioner for perennial fruits. International Journal of Agricultural Biology. 18, 293–297.
- ↑ Moore, D. http://www.davidmoore.org.uk/assets/mostly_mycology/diane_howarth/ericoid.htm
- ↑ Dighton, J. (2009). Mycorrhizae. In M. Schaechter (Eds.). Encyclopedia of Microbiology (Third Edition), Academic Press.
- ↑ Perotto, S., Daghino, S., & Martino, E. (2018). Ericoid mycorrhizal fungi and their genomes: another side to the mycorrhizal symbiosis?, New Phytologis, 220(4), 1141-1147.
- ↑ Moore, D. http://www.davidmoore.org.uk/assets/mostly_mycology/diane_howarth/ericoid.htm
- ↑ Yeh, C., Chung, K., Liang, C., & Tsai, W. (2019). New Insights into the Symbiotic Relationship between Orchids and Fungi, Applied Biosciences and Bioengineering, 9(3), 585.
- ↑ Orchid mycorrhiza, https://www.ibot.cas.cz/mykosym/en_orch.html
- ↑ Moored, D. http://www.davidmoore.org.uk/assets/mostly_mycology/diane_howarth/orchid.htm