Soil hydrology
Soil hydrology is the study of the movement and distribution of water through soil and is a subset of the larger field of Hydrogeology. Hydrologic processes within soil include infiltration, storage, drainage, redistribution, evaporation, and transpiration. The structural properties of soil (composition, porosity, etc) and the chemical properties of water regulate the flow of water and other materials through soil.
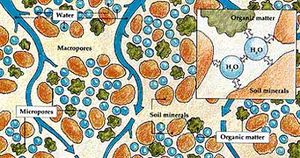
Physical Properties
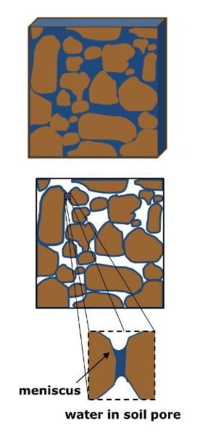
Water Distribution in Soil
Liquid water is a key component of the three-phase (solid, liquid, gas) soil system, possibly occupying 50% or more of the total soil volume under saturated conditions. Even under relatively dry conditions, water held at large tensions within soil pores occupies approximately 5–10% of the soil volume. Liquid water is held in soil under tension arising from the adhesive and cohesive forces associated with water's molecular structure.[2]
The interaction between water and the soil solid matrix is often visualized with a capillary tube model. Liquid water at the water-gas interface exhibits a meniscus. The inward pull of liquid water molecules from hydrogen bonding (cohesion) is unbalanced at the liquid-gas interface, which is referred to as surface tension. In combination with the polar attraction of water molecules for a wettable soil solid matrix (adhesion to the capillary tube wall), this cohesion creates concave curvature. Water rises in the tube to reach equilibrium between the attractive upward force at the interface and the weight of water pulling downward on the meniscus.[3] The analog of the capillary tube for soil pores also has been used to understand wetting above the water table and how management practices that alter pore size distribution, such as agricultural tillage, can affect soil's water holding capacity.
Darcy's Law
q=K(\delta)H/L
The movement of liquids through porous media was first described mathematically by Henry Darcy in 1856.[4]The equation he generated is now known as Darcy's law and is a common feature of soil biogeochemical and hydraulic models. Darcy's law when applied to soils states that the flux (q) of water is proportionate to the hydraulic conductivity of the soil (K) and the pressure gradient(\delta H ) for a given cross sectional area and length. This equation has been modified and adapted into various forms since its inception but the foundational law and all of its implications still hold true.[5]
Temperature Regulation
The prevalence of water within the soil system also drives terrestrial temperature dynamics. When liquid water enters the soil matrix, it displaces the soil gas phase. Water requires a relatively large energy input or loss (heat capacity, 4.18 kJ kg-1 K-1) to change its temperature because of strong interactions (hydrogen bonding) between dipolar water molecules. Alternately, weak inter-molecular interactions in soil gases allow soil temperatures to change readily with a small energy input or loss. Furthermore, because soil water can exist in liquid, gas (vapor) and solid (ice) phases, latent heat loss or gain from soil associated with phase change also impacts the thermal regime. In moist environments with ample soil water, water vapor loss from soil through evaporation requires a large energy input (heat of vaporization, 2449 kJ kg-1) without accompanying temperature change because of the large energy input required to disrupt the hydrogen bonds between liquid water molecules. Similarly, a large latent heat loss is required for soil freezing due to the high heat of fusion (334 kJ kg-1) associated with the more rigid, low-density structure of ice. Thermal properties of water within soil mediate environmental conditions for the biological community and regulate the thermodynamics of biogeochemical reactions.[6]
Chemical Properties
As a Solvent
The chemical properties of water govern its behavior in the environment and control many key processes occurring in soils as the aqueous phase interacts with organisms, mineral surfaces, and air spaces. As a result of its nonlinear structure and dipole moment, water has a high dielectric constant (80.1 at 20°C), which is a measure of a substance's ability to minimize the force of attraction between oppositely charged species. This unique property of water also makes it a powerful solvent, allowing it to readily dissolve ionic solids. Water acts to dissipate the attractive force of ions by forming solvation spheres around them. The polar nature of the water molecules allow them to surround and stabilize the charges of both anions and cations, preventing their association.[7][8]
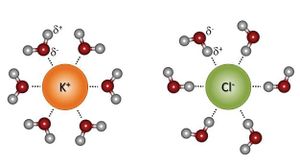
PH Regulation
Another particularly important chemical property of water that impacts processes occurring within the soil solution is that it is amphoteric, meaning that it can act as either an acid or a base. Due to its polarity, water readily undergoes ionic dissociation into protons and hydroxide ions. When it reacts with a strong base, water acts as an acid, releasing protons. When it reacts with a strong acid, water acts as a base, accepting protons.
The amphoteric behavior of water facilitates the acid-base chemistry and dictates the potential pH range of aqueous solutions, thereby imparting soil pH — a "master variable" of soils that influences soil formation, plant growth, and environmental quality.
The ability of water to stabilize charged species in solution allows it to support the flow of electrons in soils. As such, water helps mediate oxidation and reduction (redox) reactions within the soil solution. Water itself may participate in these processes, and it is a product of cellular respiration in soils. In aerobic soils, water is produced from the oxidation of carbon in organic matter for energy production by microorganisms.
References
- ↑ http://www.fao.org/3/a0072e/a0072e07.htm
- ↑ Hillel, D., 1974. Soil and water. Physical principles and processes.
- ↑ Fisher, R.A., 1926. On the capillary forces in an ideal soil; correction of formulae given by W. B. Haines. J. Agric. Sci. 16, 492–505. https://doi.org/10.1017/S0021859600007838
- ↑ Darcy, H. (1856). Les fontaines publiques de la ville de Dijon. Paris: Dalmont.
- ↑ Hillel, D., 1998. Environmental soil physics. Academic Press, San Diego, CA.
- ↑ Campbell, G. S. & Norman, J. M. An Introduction to Environmental Biophysics. Vol. second 286 Springer-Verlag, 1998.
- ↑ Sparks, D. L. Environmental Soil Chemistry. 2nd ed. Academic Press, 2003.
- ↑ Eisenberg, D. & Kauzmann, W. The Structure and Properties of Water. 296 Oxford University Press, 1969.