Lignin: Difference between revisions
| Line 3: | Line 3: | ||
== Structure == | == Structure == | ||
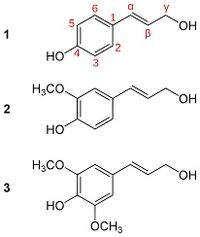
Lignin is formed by the crossing of lignols. There are three main types of lignols, conyferal alcohol, sinapyl alcohol, and paracoumaryl alcohol. These lignols are found in all plant species containing lignin, however their abundance will change according to the rigidity and type of the wood they are found in. | Lignin is formed by the crossing of lignols. There are three main types of lignols, conyferal alcohol, sinapyl alcohol, and paracoumaryl alcohol. These lignols are found in all plant species containing lignin, however their abundance will change according to the rigidity and type of the wood they are found in [2]. | ||
[[File:Lignin.jpg|200px|thumb|right|structure of the 3 main lignols]] | [[File:Lignin.jpg|200px|thumb|right|structure of the 3 main lignols]] | ||
Revision as of 11:01, 22 April 2022
Description
Lignin is a complex polymer found in the cell walls of many plant species. Lignin is especially important in the formation of cell walls in rigid and woody plant species. Lignin is incredibly rigid, allowing tree species to grow tall, while also allowing for movement of the branches in the presence of stressors such as wind and animal inhabitance. Lignin also aids in the transportation of water throughout the organism [1].
Structure
Lignin is formed by the crossing of lignols. There are three main types of lignols, conyferal alcohol, sinapyl alcohol, and paracoumaryl alcohol. These lignols are found in all plant species containing lignin, however their abundance will change according to the rigidity and type of the wood they are found in [2].
Ecological Importance
Lignin plays a crucial role in the carbon cycle. Lignin absorbs atmospheric carbon and holds it within the plant tissue. It also is one of the slowest decomposing materials of a dead tree, becoming a very high fraction of the production of humus and top soil. Only a small amount of organisms are able to decompose lignin. Fungi are known to be the greatest decomposers of lignin as they can produce an extracellular peroxidase that can kick start the decomposition of the material.
References
[1] Saake, Bodo; Lehnen, Ralph (2007). Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a15_305.pub3