Eutrophication: Difference between revisions
m The LinkTitles extension automatically added links to existing pages (<a rel="nofollow" class="external free" href="https://github.com/bovender/LinkTitles">https://github.com/bovender/LinkTitles</a>). |
|||
| (48 intermediate revisions by 5 users not shown) | |||
| Line 1: | Line 1: | ||
== Definition == | == Definition == | ||
'''Eutrophication''' | '''Eutrophication''' is the process of excessive nutrients within a body of water causing dense growth of aquatic plants and algae. Algae thrive on the excess nutrients such as nitrogen and phosphorus introduced into a body of water. This nutrient pollution is often from [[agriculture]], stormwater and wastewater runoff into waterways [1]. As the algae die and decompose, available oxygen within the water is consumed. The lack of dissolved oxygen results in "dead zones" causing the marine life to die or move away from the area [2]. | ||
== Causes == | == Causes == | ||
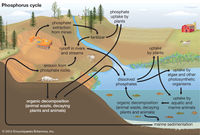
Eutrophication can occur naturally over many years, but it is accelerated by human activities (a phenomenon known as cultural eutrophication). Of these anthropogenic activities, agricultural runoff from fertilizers and the disposal of wastewater are some of the leading causes of cultural eutrophication. Typically occurring phosphorus and nitrogen concentrations are low enough to prevent aquatic plants or algae from growing out of control. However, human activities can rapidly increase these natural levels. Fertilizers are designed to be rich in phosphorus (in the form of phosphates) or nitrogen (concentrations vary between plants) to aid in the growth of crops and produce higher yields. Natural events, such as precipitation, can carry these chemicals via runoff to ponds or lakes, resulting in an exponential increase of the local plant and algae populations, as their limiting factor for growth (nutrients) is no longer an issue [10]. | |||
[[File:126077-050-117592F5.jpg|200px|thumb|Diagram of Phosphorus Cycle|right]] | |||
== Consequences == | == Consequences == | ||
The rapid increase in plant and algae populations produces Harmful Algal Blooms (HABs) that pose serious threats to more than just the quality of water. Some HABs release toxins that can kill fish, birds, and mammals that consume the effected water causing detriment to the entire ecosystem. In extreme cases, these toxins may cause severe human illness or death [9]. While not all algal species are toxic, algae reduce the levels of available dissolved oxygen in a water body when they die and undergo [[decomposition]]. In turn, this lack of available oxygen within the water affects the ability of many fish, as well as other aquatic [[animals]] and plants, to survive within the ecosystem. Biodiversity can be reduced as a result, leading to significant changes within the local food chain. Furthermore, dead algae and plant material will often accumulate at the bottom of the water body, where it will undergo anaerobic digestion. This anaerobic digestion releases greenhouse gases like methane and carbon dioxide, which contribute to global warming. Due to the large-scale displacement of sediment, cultural eutrophication is extremely detrimental to the integrity of terrestrial [[soil]] habitats as well [3]. [[File:eutrofizzazione.jpg|300px|thumb|right|Harmful Algal Bloom]] | |||
== Prevention & Reversal == | == Prevention & Reversal == | ||
Despite its ability to devastate marine habitats, cultural eutrophication can be slowed and | Despite its ability to devastate marine habitats, cultural eutrophication can be slowed and potentially reversed. There have been phosphorus removal measures taken in Finland which had a 90% success rate [4]. Others have proposed encouraging the growth of shellfish populations because these [[organisms]] take nitrogen out of the water, acting as natural filters and reducing the likelihood of algal blooms. Reducing the harmful effects of non-point source pollution is one of the most widely supported strategies for slowing the rates of eutrophication. Some of these methods include riparian buffer zones and organic farming [5]. | ||
'''Buffer Zones''' [[File:220px-Riparian_buffer_on_Bear_Creek_in_Story_County,_Iowa.JPG|200px|thumb| | '''Buffer Zones''' [[File:220px-Riparian_buffer_on_Bear_Creek_in_Story_County,_Iowa.JPG|200px|thumb|Riparian Buffer on Bear Creek in Story County, Iowa|right]] | ||
Buffer | |||
Buffer zones, specifically riparian buffer zones, are meant to act as an initial filter to prevent non-point source pollution from contaminating a water source [6]. Rather than being a man-made structure, a riparian buffer zone is an area of natural vegetation along the bank of the stream or river, like that of a mangrove forest in Southern Florida [7]. | |||
'''Organic Farming''' | '''Organic Farming''' | ||
Organic farming is said to be another very effective method | Organic farming is said to be another very effective method for slowing the rates of anthropogenic eutrophication due to the non-existent use of synthetic, nitrogen-rich fertilizers. A study found that fields that were fertilized through organic means were not nearly as harmful as more conventional farming practices in terms of nitrate leaching [8]. | ||
== References == | == References == | ||
1. | 1. U.S. Environmental Protection Agency. (n.d.). "Sources and solutions". U.S. Environmental Protection Agency. https://www.epa.gov/nutrientpollution/sources-and-solutions. | ||
2. National Oceanic and Atmospheric Administration. (n.d.). "Dead Zone". National Oceanic and Atmospheric Administration. https://oceanservice.noaa.gov/facts/deadzone.html. | |||
3. Tittmann, A. (n.d.). "Climate gases from water bodies". IGB. URL https://www.igb-berlin.de/en/news/climate-gases-water-bodies. | |||
4. Räike, A., Pietiläinen, O. -P., Rekolainen, S., Kauppila, P., Pitkänen, H., Niemi, J., Raateland, A., Vuorenmaa, J. (2003). "Trends of phosphorus, nitrogen and chlorophyll concentrations in Finnish rivers and lakes in 1975–2000". Science of the Total Environment. 310 (1–3): 47–59. Bibcode:2003ScTEn.310...47R. doi:10.1016/S0048-9697(02)00622-8. PMID 12812730. | |||
5. Kroeger, T. (2012). "Dollars and Sense: Economic Benefits and Impacts from two Oyster Reef Restoration Projects in the Northern Gulf of Mexico Archived 2016-03-04 at the Wayback Machine". TNC Report. | |||
6. Carpenter, S.R., Caraco, N.F., Smith, V.H. (1998). "Nonpoint pollution of surface waters with phosphorus and nitrogen". Ecological Applications. 8 (3): 559–568. doi:10.2307/2641247. hdl:1813/60811. JSTOR 2641247. | |||
7. "Importance of Riparian Buffers". (2019). https://dep.wv.gov/WWE/getinvolved/sos/Pages/RiparianMagic.aspx. | |||
8. Kramer, S. B. (2006). "Reduced nitrate leaching and enhanced denitrifier activity and efficiency in organically fertilized soils". Proceedings of the National Academy of Sciences. 103 (12): 4522–4527. Bibcode:2006PNAS..103.4522K. doi:10.1073/pnas.0600359103. PMC 1450204. PMID 16537377. | |||
9. Morris, J. G. (1990). "Harmful algal blooms: an emerging public health problem with possible links to human stress on the environment". Annual review of Energy and the Environment. 24: 367-390. | |||
10. Michael F. C. (2013). "Eutrophication Causes, Consequences, and Controls in Aquatic Ecosystems". Nature Education. | |||
Revision as of 16:46, 10 March 2025
Definition
Eutrophication is the process of excessive nutrients within a body of water causing dense growth of aquatic plants and algae. Algae thrive on the excess nutrients such as nitrogen and phosphorus introduced into a body of water. This nutrient pollution is often from agriculture, stormwater and wastewater runoff into waterways [1]. As the algae die and decompose, available oxygen within the water is consumed. The lack of dissolved oxygen results in "dead zones" causing the marine life to die or move away from the area [2].
Causes
Eutrophication can occur naturally over many years, but it is accelerated by human activities (a phenomenon known as cultural eutrophication). Of these anthropogenic activities, agricultural runoff from fertilizers and the disposal of wastewater are some of the leading causes of cultural eutrophication. Typically occurring phosphorus and nitrogen concentrations are low enough to prevent aquatic plants or algae from growing out of control. However, human activities can rapidly increase these natural levels. Fertilizers are designed to be rich in phosphorus (in the form of phosphates) or nitrogen (concentrations vary between plants) to aid in the growth of crops and produce higher yields. Natural events, such as precipitation, can carry these chemicals via runoff to ponds or lakes, resulting in an exponential increase of the local plant and algae populations, as their limiting factor for growth (nutrients) is no longer an issue [10].
Consequences
The rapid increase in plant and algae populations produces Harmful Algal Blooms (HABs) that pose serious threats to more than just the quality of water. Some HABs release toxins that can kill fish, birds, and mammals that consume the effected water causing detriment to the entire ecosystem. In extreme cases, these toxins may cause severe human illness or death [9]. While not all algal species are toxic, algae reduce the levels of available dissolved oxygen in a water body when they die and undergo decomposition. In turn, this lack of available oxygen within the water affects the ability of many fish, as well as other aquatic animals and plants, to survive within the ecosystem. Biodiversity can be reduced as a result, leading to significant changes within the local food chain. Furthermore, dead algae and plant material will often accumulate at the bottom of the water body, where it will undergo anaerobic digestion. This anaerobic digestion releases greenhouse gases like methane and carbon dioxide, which contribute to global warming. Due to the large-scale displacement of sediment, cultural eutrophication is extremely detrimental to the integrity of terrestrial soil habitats as well [3].

Prevention & Reversal
Despite its ability to devastate marine habitats, cultural eutrophication can be slowed and potentially reversed. There have been phosphorus removal measures taken in Finland which had a 90% success rate [4]. Others have proposed encouraging the growth of shellfish populations because these organisms take nitrogen out of the water, acting as natural filters and reducing the likelihood of algal blooms. Reducing the harmful effects of non-point source pollution is one of the most widely supported strategies for slowing the rates of eutrophication. Some of these methods include riparian buffer zones and organic farming [5].
Buffer Zones

Buffer zones, specifically riparian buffer zones, are meant to act as an initial filter to prevent non-point source pollution from contaminating a water source [6]. Rather than being a man-made structure, a riparian buffer zone is an area of natural vegetation along the bank of the stream or river, like that of a mangrove forest in Southern Florida [7].
Organic Farming
Organic farming is said to be another very effective method for slowing the rates of anthropogenic eutrophication due to the non-existent use of synthetic, nitrogen-rich fertilizers. A study found that fields that were fertilized through organic means were not nearly as harmful as more conventional farming practices in terms of nitrate leaching [8].
References
1. U.S. Environmental Protection Agency. (n.d.). "Sources and solutions". U.S. Environmental Protection Agency. https://www.epa.gov/nutrientpollution/sources-and-solutions.
2. National Oceanic and Atmospheric Administration. (n.d.). "Dead Zone". National Oceanic and Atmospheric Administration. https://oceanservice.noaa.gov/facts/deadzone.html.
3. Tittmann, A. (n.d.). "Climate gases from water bodies". IGB. URL https://www.igb-berlin.de/en/news/climate-gases-water-bodies.
4. Räike, A., Pietiläinen, O. -P., Rekolainen, S., Kauppila, P., Pitkänen, H., Niemi, J., Raateland, A., Vuorenmaa, J. (2003). "Trends of phosphorus, nitrogen and chlorophyll concentrations in Finnish rivers and lakes in 1975–2000". Science of the Total Environment. 310 (1–3): 47–59. Bibcode:2003ScTEn.310...47R. doi:10.1016/S0048-9697(02)00622-8. PMID 12812730.
5. Kroeger, T. (2012). "Dollars and Sense: Economic Benefits and Impacts from two Oyster Reef Restoration Projects in the Northern Gulf of Mexico Archived 2016-03-04 at the Wayback Machine". TNC Report.
6. Carpenter, S.R., Caraco, N.F., Smith, V.H. (1998). "Nonpoint pollution of surface waters with phosphorus and nitrogen". Ecological Applications. 8 (3): 559–568. doi:10.2307/2641247. hdl:1813/60811. JSTOR 2641247.
7. "Importance of Riparian Buffers". (2019). https://dep.wv.gov/WWE/getinvolved/sos/Pages/RiparianMagic.aspx.
8. Kramer, S. B. (2006). "Reduced nitrate leaching and enhanced denitrifier activity and efficiency in organically fertilized soils". Proceedings of the National Academy of Sciences. 103 (12): 4522–4527. Bibcode:2006PNAS..103.4522K. doi:10.1073/pnas.0600359103. PMC 1450204. PMID 16537377.
9. Morris, J. G. (1990). "Harmful algal blooms: an emerging public health problem with possible links to human stress on the environment". Annual review of Energy and the Environment. 24: 367-390.
10. Michael F. C. (2013). "Eutrophication Causes, Consequences, and Controls in Aquatic Ecosystems". Nature Education.