Eutrophication
Definition
Eutrophication, sometimes known as hypertrophication, is the process by which a body of water becomes enriched in dissolved nutrients (such as phosphates) through the intense breakdown of soil sediments that stimulate the growth of aquatic plant life. This will usually lead to the depletion of dissolved oxygen which is known as hypoxia. [1] This phenomena is perfectly fine when it happens at normal rates, but not at the rates that we are currently witnessing.

Causes
While eutrophication is usually a natural process that takes place over the course of hundreds of years, it has in recent decades been negatively influenced and accelerated due to human influence. Naturally occurring eutrophication happens on geologic time scales, [3] but as mentioned, that is no longer the case in most places of the world due to anthropogenic or cultural eutrophication. Cultural eutrophication occurs through either non-point source or point source pollution at the hands of humans. [2] Examples of point-source or non-point source pollution that increase the rates of eutrophication can include, detergents, fertilizers, or sewage which can come from almost anywhere whether it be parking lots and roads or agricultural fields. [3][4] The most problematic of these tends to be the fertilizers that are used primarily in the cultivation of agricultural fields and grass lawns. Not to mention, clearing of land as well as the building of cities and towns leads to sediment runoff which worsens the rates that phosphates and nitrates make their way into bodies of water. [5]
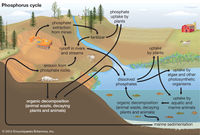
Consequences
Because of this sudden flux of nutrients, plant life, especially algae, are permitted to flourish. It is when these organisms die though that they become decomposed and it is in this decomposition process that oxygen is consumed which in turn reduces the oxygen concentration in the water. The lack of oxygen in the water of course greatly reduces the number of fish and other animals in said aquatic ecosystem as well as the overall biodiversity. To make matters even worse, the dead algae and other plant material can settle at the bottom of the body of water where it will undergo anaerobic digestion. This anaerobic digestion releases greenhouse gases like that of methane and carbon dioxide which are incredibly harmful to the atmosphere and the Earth as a whole. [6]
Prevention & Reversal
Despite its ability to devastate marine habitats, cultural eutrophication can be slowed and even reversed. There have been phosphorus removal measures taken in Finland which have been said to have had a 90% success rate. [7]
References
1. Eutrophication. (n.d.). . Merriam-Webster. https://www.merriam-webster.com/dictionary/eutrophication.
2. Chislock, M. F., Doster, E., Zitomer, R. A. & Wilson, A. E. (2013) Eutrophication: Causes, Consequences, and Controls in Aquatic Ecosystems. Nature Education Knowledge 4(4):10
3. Callisto, Marcos; Molozzi, Joseline and Barbosa, José Lucena Etham (2014) "Eutrophication of Lakes" in A. A. Ansari, S. S. Gill (eds.), Eutrophication: Causes, Consequences and Control, Springer Science+Business Media Dordrecht. doi:10.1007/978-94-007-7814-6_5. ISBN 978-94-007-7814-6.
4. Muir, P., 2012. Eutrophication [WWW Document]. Oregon State University. URL http://people.oregonstate.edu/~muirp/eutrophi.htm
5. Schindler, David W., Vallentyne, John R. (2008). The Algal Bowl: Overfertilization of the World's Freshwaters and Estuaries, University of Alberta Press, ISBN 0-88864-484-1.
6. Tittmann, A., n.d. Climate gases from water bodies [WWW Document]. IGB. URL https://www.igb-berlin.de/en/news/climate-gases-water-bodies
7. Räike, A.; Pietiläinen, O. -P.; Rekolainen, S.; Kauppila, P.; Pitkänen, H.; Niemi, J.; Raateland, A.; Vuorenmaa, J. (2003). "Trends of phosphorus, nitrogen and chlorophyll a concentrations in Finnish rivers and lakes in 1975–2000". Science of the Total Environment. 310 (1–3): 47–59. Bibcode:2003ScTEn.310...47R. doi:10.1016/S0048-9697(02)00622-8. PMID 12812730.