Extracellular polymeric substance: Difference between revisions
No edit summary |
No edit summary |
||
| Line 12: | Line 12: | ||
===Chemical reactions=== | ===Chemical reactions=== | ||
EPS provides a stable interface for soil microbes to chemically work on attached substrate and suspended materials. In addition EPS | EPS provides a stable interface for soil microbes to chemically work on attached substrate and suspended materials. In addition EPS can act as a store of carbon and other nutrients. The binding nature of EPS also helps reduce nutrient loss in soils from runoff.<ref>Lin, D., Ma, W., Jin, Z., Wang, Y., Huang, Q., Cai, P., 2016. Interactions of EPS with soil minerals: A combination study by ITC and CLSM. Colloids and Surfaces B: Biointerfaces 138, 10–16. https://doi.org/10.1016/j.colsurfb.2015.11.026</ref> | ||
[[File:fmicb-09-01636-g001.jpg|thumb|right|Theoretical functions of soil EPS<ref name=Costa_2018 />]] | [[File:fmicb-09-01636-g001.jpg|thumb|right|Theoretical functions of soil EPS<ref name=Costa_2018 />]] | ||
===Hydrology=== | ===Hydrology=== | ||
EPS resists evaporation by binding | EPS affects evaporation and flow of water directly and through alterations to the functional soil structure. EPS resists evaporation and slows flow by absorbing and binding water tightly. Also, the biofilm structures formed with EPS can cause bioclogging of pores which blocks evaporation and mass flow of water by reducing the hydraulic conductivity<ref name=Deng_2015 /><ref name=Or_2007 />. This slows the overall rate of change in soil moisture content making for a more stable environment. | ||
===[[Soil Structures]]=== | ===[[Soil Structures]]=== | ||
EPS production fills | EPS production fills pore space which reduces the effective [[porosity]] of the soil. Also, the swelling shrinking actions of EPS water intake and loss can alter the pore space but there remains a lack of literature differentiating this effect in bulk soil<ref name=Deng_2015 />. | ||
EPS plays a key role in soil [[aggregate formation]] by working as a cementing agent. This has the added effect of reducing soil slaking and increasing overall stability. This added stability can lower erosion rates and decrease nutrient runoff. | EPS plays a key role in soil [[aggregate formation]] by working as a cementing agent. This has the added effect of reducing soil slaking and increasing overall stability. This added stability can lower erosion rates and decrease nutrient runoff. | ||
===Plants=== | ===Plants=== | ||
EPS | EPS play a role in holding microbes to roots or in the [[rhizosphere]] and act as the medium for symbiotic microbes to exchange nutrients in exchange for root exudates<ref name=Costa_2018 />. The microbes, through the effects of EPS and release of nutrients, stimulate the the exudate release by the roots which introduces fresh carbon sources to the rhizosphere. There is also evidence to suggest EPS assists in salinity tolerance for some plants.<ref name=Ashraf_2004>Ashraf, M., Hasnain, S., Berge, O., Mahmood, T., 2004. Inoculating wheat seedlings with exopolysaccharide-producing bacteria restricts sodium uptake and stimulates plant growth under salt stress. Biol Fertil Soils 40. https://doi.org/10.1007/s00374-004-0766-y</ref> | ||
===Agriculture=== | ===Agriculture=== | ||
There is growing interest in using EPS producing bacteria | There is growing interest in using EPS producing bacteria in agricultural settings. EPS improves soil particle aggregation which is an issue common to traditional agricultural practices in may parts of the world. Also, EPS producing bacteria improve nutrient availability to plants, increase water stability, and stimulate root growth.<ref name=Costa_2018 /> | ||
==Analysis methods== | ==Analysis methods== | ||
Cation exchange resin (CER) is currently considered the best method for accurately extracting EPS from soils.<ref name=Redmile-Gordon_2014>Redmile-Gordon, M.A., Brookes, P.C., Evershed, R.P., Goulding, K.W.T., Hirsch, P.R., 2014. Measuring the soil-microbial interface: Extraction of extracellular polymeric substances (EPS) from soil biofilms. Soil Biology and Biochemistry 72, 163–171.</ref> | Cation exchange resin (CER) extraction is currently considered the best method for accurately extracting EPS from soils.<ref name=Redmile-Gordon_2014>Redmile-Gordon, M.A., Brookes, P.C., Evershed, R.P., Goulding, K.W.T., Hirsch, P.R., 2014. Measuring the soil-microbial interface: Extraction of extracellular polymeric substances (EPS) from soil biofilms. Soil Biology and Biochemistry 72, 163–171.</ref>This method shows the highest efficency with the least amount of modification to the original EPS composition when compared to other methods. This method works by replacing cations in the material being sampled. This destabilizes the structure and allows for separation through filtration and/or centrifuge. | ||
Latest revision as of 23:49, 6 May 2021

Overview
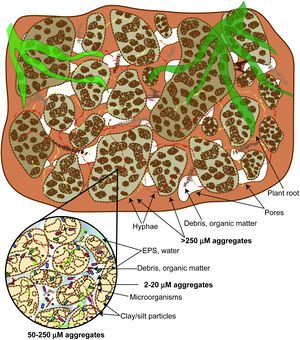
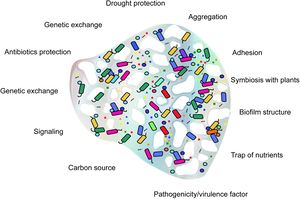
Extracellular polymeric substances (EPS) are a group of substances exuded by microorganisms in order to form biofilms. The major components are extracellular polysaccharides (sometimes also called EPS), proteins, DNA, enzymes, lipids, and other biopolymers[2]. EPS accounts for 90% of biofilm dry mass and constitutes the matrix of the biofilm[2]. Biofilms are micro-habitats that keep microbes attached to a substrate[3], provide protection from desiccation[4][5], and play a key role in nutrient cycling[6]. EPS is known to alter the structure [3][7], hydrology [5][8][9][10], and composition [7][11][12] of soils. It is important in aggregate formation and acts as an interface between soil and plant interactions with microbes. At the soil interface EPS facilitates chemical reactions and transportation of nutrients. At the plant interface EPS acts as the exchange media through which plant exudates and microbial secretions can be exchanged.
Functions
Habitat stability
The suspected primary role of EPS is to create stable habitat bound to a desired substrate[6]. EPS is the matrix structure of biofilms. Biofilms are very effective at retaining water in soils even with very negative water potentials.[9] This allows microbes to resist desiccation during drought periods. EPS can also stabilize pH and reduce the amount of nutrients lost to runoff by binding to them.
Chemical reactions
EPS provides a stable interface for soil microbes to chemically work on attached substrate and suspended materials. In addition EPS can act as a store of carbon and other nutrients. The binding nature of EPS also helps reduce nutrient loss in soils from runoff.[13]
Hydrology
EPS affects evaporation and flow of water directly and through alterations to the functional soil structure. EPS resists evaporation and slows flow by absorbing and binding water tightly. Also, the biofilm structures formed with EPS can cause bioclogging of pores which blocks evaporation and mass flow of water by reducing the hydraulic conductivity[10][9]. This slows the overall rate of change in soil moisture content making for a more stable environment.
Soil Structures
EPS production fills pore space which reduces the effective porosity of the soil. Also, the swelling shrinking actions of EPS water intake and loss can alter the pore space but there remains a lack of literature differentiating this effect in bulk soil[10]. EPS plays a key role in soil aggregate formation by working as a cementing agent. This has the added effect of reducing soil slaking and increasing overall stability. This added stability can lower erosion rates and decrease nutrient runoff.
Plants
EPS play a role in holding microbes to roots or in the rhizosphere and act as the medium for symbiotic microbes to exchange nutrients in exchange for root exudates[12]. The microbes, through the effects of EPS and release of nutrients, stimulate the the exudate release by the roots which introduces fresh carbon sources to the rhizosphere. There is also evidence to suggest EPS assists in salinity tolerance for some plants.[14]
Agriculture
There is growing interest in using EPS producing bacteria in agricultural settings. EPS improves soil particle aggregation which is an issue common to traditional agricultural practices in may parts of the world. Also, EPS producing bacteria improve nutrient availability to plants, increase water stability, and stimulate root growth.[12]
Analysis methods
Cation exchange resin (CER) extraction is currently considered the best method for accurately extracting EPS from soils.[15]This method shows the highest efficency with the least amount of modification to the original EPS composition when compared to other methods. This method works by replacing cations in the material being sampled. This destabilizes the structure and allows for separation through filtration and/or centrifuge.
References
- ↑ Aalexopo, https://commons.wikimedia.org/wiki/File:Biofilm_Formation.jpg.CC BY-SA 3.0
- ↑ 2.0 2.1 Flemming, H.-C., Wingender, J., 2010. The biofilm matrix. Nat Rev Microbiol 8, 623–633. https://doi.org/10.1038/nrmicro2415
- ↑ 3.0 3.1 Azeredo, J., Oliveira, R., 2000. The role of exopolymers in the attachment of sphingomonas paucimobilis. Biofouling 16, 59–67. https://doi.org/10.1080/08927010009378430
- ↑ Tamaru, Y., Takani, Y., Yoshida, T., Sakamoto, T., 2005. Crucial Role of Extracellular Polysaccharides in Desiccation and Freezing Tolerance in the Terrestrial Cyanobacterium Nostoc commune. AEM 71, 7327–7333. https://doi.org/10.1128/AEM.71.11.7327-7333.2005
- ↑ 5.0 5.1 Roberson, E.B., Firestone, M.K., 1992. Relationship between Desiccation and Exopolysaccharide Production in a Soil Pseudomonas sp. Applied and Environmental Microbiology 58, 1284–1291. https://doi.org/10.1128/AEM.58.4.1284-1291.1992
- ↑ 6.0 6.1 Flemming, H.-C., Wingender, J., Szewzyk, U., Steinberg, P., Rice, S.A., Kjelleberg, S., 2016. Biofilms: an emergent form of bacterial life. Nat Rev Microbiol 14, 563–575. https://doi.org/10.1038/nrmicro.2016.94
- ↑ 7.0 7.1 Mager, D.M., Thomas, A.D., 2011. Extracellular polysaccharides from cyanobacterial soil crusts: A review of their role in dryland soil processes. Journal of Arid Environments 75, 91–97. https://doi.org/10.1016/j.jaridenv.2010.10.001
- ↑ Morales, V.L., Parlange, J.-Y., Steenhuis, T.S., 2010. Are preferential flow paths perpetuated by microbial activity in the soil matrix? A review. Journal of Hydrology 393, 29–36. https://doi.org/10.1016/j.jhydrol.2009.12.048
- ↑ 9.0 9.1 9.2 r, D., Phutane, S., Dechesne, A., 2007. Extracellular Polymeric Substances Affecting Pore-Scale Hydrologic Conditions for Bacterial Activity in Unsaturated Soils. Vadose Zone Journal 6, 298–305. https://doi.org/10.2136/vzj2006.0080
- ↑ 10.0 10.1 10.2 Deng, J., Orner, E.P., Chau, J.F., Anderson, E.M., Kadilak, A.L., Rubinstein, R.L., Bouchillon, G.M., Goodwin, R.A., Gage, D.J., Shor, L.M., 2015. Synergistic effects of soil microstructure and bacterial EPS on drying rate
- ↑ Gunina, A., Kuzyakov, Y., 2015. Sugars in soil and sweets for microorganisms: Review of origin, content, composition and fate. Soil Biology and Biochemistry 90, 87–100. https://doi.org/10.1016/j.soilbio.2015.07.021
- ↑ 12.0 12.1 12.2 12.3 Costa, O.Y.A., Raaijmakers, J.M., Kuramae, E.E., 2018. Microbial Extracellular Polymeric Substances: Ecological Function and Impact on Soil Aggregation. Front. Microbiol. 9, 1636. https://doi.org/10.3389/fmicb.2018.01636
- ↑ Lin, D., Ma, W., Jin, Z., Wang, Y., Huang, Q., Cai, P., 2016. Interactions of EPS with soil minerals: A combination study by ITC and CLSM. Colloids and Surfaces B: Biointerfaces 138, 10–16. https://doi.org/10.1016/j.colsurfb.2015.11.026
- ↑ Ashraf, M., Hasnain, S., Berge, O., Mahmood, T., 2004. Inoculating wheat seedlings with exopolysaccharide-producing bacteria restricts sodium uptake and stimulates plant growth under salt stress. Biol Fertil Soils 40. https://doi.org/10.1007/s00374-004-0766-y
- ↑ Redmile-Gordon, M.A., Brookes, P.C., Evershed, R.P., Goulding, K.W.T., Hirsch, P.R., 2014. Measuring the soil-microbial interface: Extraction of extracellular polymeric substances (EPS) from soil biofilms. Soil Biology and Biochemistry 72, 163–171.