Monocots: Difference between revisions
No edit summary |
No edit summary |
||
| Line 7: | Line 7: | ||
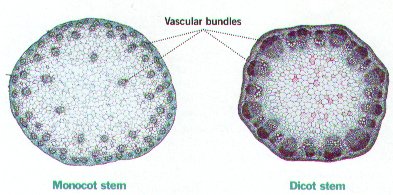
The identifying feature of a monocot is a trimerious-pentacyclic flower design. This design consists of six tepals in two whorls, six stamens in two whorls, and three carpels. These features are virtually absent from earlier angiosperms (Remizowa, Sokoloff, & Rudall, 2010). Floral parts in monocots typically occur in threes (Robinson, 2016). Monocots are also known to have leaves with parallel venation, the veins are arranged parallel to one another and do not join other veins. Monocotyledons differ from the other main group of angiosperms, eudicots, in their vascular structure. Monocots have primary vascular bundles containing both phloem and xylem in a scattered arrangement, known as an atactostele. There is no differentiation between cortical and stellar regions in monocots. This contrasts eudicots, which have a ring like vascular bundle shape with a distinct phloem and xylem, differentiated by the cortex and stele (Zimmermann and Tomlinson, 1972). Root systems in monocots are characterized by a radical which aborts at an early stage. Since the first root that emerges dies, a central taproot forms and roots grows adventitiously (Chase, 2004). Adventitious roots sprout from shoot tissues near the base of the monocot. Monocots also lack a cambium, which allows for growth in diameter with height (Chase, 2004). Due to the lack of cambium, there is a limit on how tall shoots can grow and many monocots tend to be herbaceous. However, some monocots are able to reach great height, length and mass such as agaves, palms, and bamboos. | The identifying feature of a monocot is a trimerious-pentacyclic flower design. This design consists of six tepals in two whorls, six stamens in two whorls, and three carpels. These features are virtually absent from earlier angiosperms (Remizowa, Sokoloff, & Rudall, 2010). Floral parts in monocots typically occur in threes (Robinson, 2016). Monocots are also known to have leaves with parallel venation, the veins are arranged parallel to one another and do not join other veins. Monocotyledons differ from the other main group of angiosperms, eudicots, in their vascular structure. Monocots have primary vascular bundles containing both phloem and xylem in a scattered arrangement, known as an atactostele. There is no differentiation between cortical and stellar regions in monocots. This contrasts eudicots, which have a ring like vascular bundle shape with a distinct phloem and xylem, differentiated by the cortex and stele (Zimmermann and Tomlinson, 1972). Root systems in monocots are characterized by a radical which aborts at an early stage. Since the first root that emerges dies, a central taproot forms and roots grows adventitiously (Chase, 2004). Adventitious roots sprout from shoot tissues near the base of the monocot. Monocots also lack a cambium, which allows for growth in diameter with height (Chase, 2004). Due to the lack of cambium, there is a limit on how tall shoots can grow and many monocots tend to be herbaceous. However, some monocots are able to reach great height, length and mass such as agaves, palms, and bamboos. | ||
[[File: | [[File:monodistems.jpg]] | ||
=Reproductive Structures= | =Reproductive Structures= | ||
Revision as of 01:10, 7 March 2018
Monocots
Monocots make up one of the largest groups of angiosperms, or flowering plants, comprising a total of twenty-five percent of all angiosperms (Fay, 2013). The term monocot, stems from most membering plants having one seed leaf, known as a cotyledon. There are nearly 60,000 different species of monocots and together they form a monophyletic group (Bremer, 2000). Famous monocot families include Orchidaceae (orchids), the largest known plant family, Iridaceae (irises), Arecaceae (palms) and more (Tang et al., 2017). Monocots are of great economic and cultural importance around the world (Tang et al., 2017).
Physical Structure
The identifying feature of a monocot is a trimerious-pentacyclic flower design. This design consists of six tepals in two whorls, six stamens in two whorls, and three carpels. These features are virtually absent from earlier angiosperms (Remizowa, Sokoloff, & Rudall, 2010). Floral parts in monocots typically occur in threes (Robinson, 2016). Monocots are also known to have leaves with parallel venation, the veins are arranged parallel to one another and do not join other veins. Monocotyledons differ from the other main group of angiosperms, eudicots, in their vascular structure. Monocots have primary vascular bundles containing both phloem and xylem in a scattered arrangement, known as an atactostele. There is no differentiation between cortical and stellar regions in monocots. This contrasts eudicots, which have a ring like vascular bundle shape with a distinct phloem and xylem, differentiated by the cortex and stele (Zimmermann and Tomlinson, 1972). Root systems in monocots are characterized by a radical which aborts at an early stage. Since the first root that emerges dies, a central taproot forms and roots grows adventitiously (Chase, 2004). Adventitious roots sprout from shoot tissues near the base of the monocot. Monocots also lack a cambium, which allows for growth in diameter with height (Chase, 2004). Due to the lack of cambium, there is a limit on how tall shoots can grow and many monocots tend to be herbaceous. However, some monocots are able to reach great height, length and mass such as agaves, palms, and bamboos.
Reproductive Structures
A monocot seed is comprised of the plant embryo, cotyledon and endosperm. The embryo will consume the endosperm and the cotyledon will transfer the stored energy to the embryo (Sreenivasulu & Wobus, 2013). As the embryo develops it sends a primary root out of the seed coating and into the soil. A primary leaf is then pushed up through the seed coating to the surface. This process differs from dicots in that a dicot’s primary roots will grow towards the soil surface initially.
DNA
Based on recent data, monocots have been identified to be a monophyletic group that diverged from other angiosperms over a million years BP, during the Cretaceous era (Bremer, 2000). Advances in molecular systematics have enabled eight major groups of monocot to be identified. Moving from the most basal, these groups include Acorus, Alismatales, and a polytomy of six remaining clades known as Japonolirion, Dioscoreales, Pandanales, Liliales, Asparagales, and a commelinoid clade with subclades, Arecaceae, Zinigiberales, and Poales (Furness & Rudall, 2004). While monocots were originally named due to having a single cotyledon, 18s rDNA evidence has shown that a stronger uniting factor among monocots is pollen grain type. Through the use of molecular systematics, it has been found that taxa with uniaperturate pollen form a grade at the base of the angiosperm tree (Soltis et al., 2000). Nearly all monocots have been identified to have uniaperturate pollen grains which distinguishes them from a large eudicot clade with taxa having primarily triaperturate pollen (Soltis, Bell, Kim, & Soltis, 2008)
Uses
Monocots are of great cultural and economic importance. Grasses, in particular, are monocots which are of vital importance to both humans and animals (Poaceae) (Fay, 2013). Monocots are ubiquitous in the diet of humans. Grains such as rice, wheat, and barley are all monocots (Anderson & Janßen, 2009). Fruits such as plantains, coconuts, pineapples, and dates fall under the monocot clade. In addition to the monocot’s dietary use, some monocots are used medicinally. The genus Dioscorea is responsible for producing diosgenin, a steroid-like compound that aids in making progesterone for many contraceptives. Indigenous communities around the world rely on monocots for medicinal purposes (Mahomoodally, 2013; Maroyi, 2013; Uprety, Asselin, Dhakal, & Julien, 2012). Other monocots used by humans include the sedge family (Cyperaceae), palms, lilies, bromeliads, skunk cabbage, and philodendron (Robinson, 2016).
References
Bremer, K. (2000). Early Cretaceous Lineages of Monocot Flowering Plants. Proceedings of the National Academy of Sciences of the United States of America, 97(9), 4707–4711. https://doi.org/10.1073/pnas.080421597
Chase, M. W. (2004). Monocot relationships: An overview. American Journal of Botany. https://doi.org/10.3732/ajb.91.10.1645
Fay, M. F. (2013). Monocots. Botanical Journal of the Linnean Society, 172(1), 1–4. https://doi.org/10.1111/boj.12052
Furness, C. A., & Rudall, P. J. (2004). Pollen aperture evolution - A crucial factor for eudicot success? Trends in Plant Science. https://doi.org/10.1016/j.tplants.2004.01.001
Mahomoodally, M. F. (2013). Traditional medicines in Africa: An appraisal of ten potent African medicinal plants. Evidence-Based Complementary and Alternative Medicine. https://doi.org/10.1155/2013/617459
Maroyi, A. (2013). Traditional use of medicinal plants in south-central Zimbabwe: Review and perspectives. Journal of Ethnobiology and Ethnomedicine. https://doi.org/10.1186/1746-4269-9-31
Remizowa, M. V., Sokoloff, D. D., & Rudall, P. J. (2010). Evolutionary History of the Monocot Flower 1. Annals of the Missouri Botanical Garden, 97(4), 617–645. https://doi.org/10.3417/2009142
Soltis, D. E., Bell, C. D., Kim, S., & Soltis, P. S. (2008). Origin and early evolution of angiosperms. Annals of the New York Academy of Sciences. https://doi.org/10.1196/annals.1438.005
Soltis, D. E., Soltis, P. S., Chase, M. W., Mort, M. E., Albach, D. C., Zanis, M., … Farris, J. S. (2000). Angiosperm phylogeny inferred from 18S rDNA, rbcL, and atpB sequences. Botanical Journal of the Linnean Society, 133(4), 381–461. https://doi.org/10.1006/bojl.2000.0380
Sreenivasulu, N., & Wobus, U. (2013). Seed-Development Programs: A Systems Biology–Based Comparison Between Dicots and Monocots. Annual Review of Plant Biology, 64(1), 189–217. https://doi.org/10.1146/annurev-arplant-050312-120215
Tang, C. Q., Orme, C. D. L., Bunnefeld, L., Jones, F. A., Powell, S., Chase, M. W., … Savolainen, V. (2017). Global monocot diversification: geography explains variation in species richness better than environment or biology. Botanical Journal of the Linnean Society, 183(1), 1–15. https://doi.org/10.1111/boj.12497
Uprety, Y., Asselin, H., Dhakal, A., & Julien, N. (2012). Traditional use of medicinal plants in the boreal forest of Canada: Review and perspectives. Journal of Ethnobiology and Ethnomedicine. https://doi.org/10.1186/1746-4269-8-7