Hammerhead Worm: Difference between revisions
No edit summary |
No edit summary |
||
| Line 41: | Line 41: | ||
== Impact as an Invasive Species == | == Impact as an Invasive Species == | ||
[[File:invasionmap.jpg|right|600px|thumb|A map of where ''Bipalium'' has been found in the United States | [[File:invasionmap.jpg|right|600px|thumb|A map of where ''Bipalium'' has been found in the United States [6]]] | ||
Since its introduction to the United States in the 1800s, ''R. cathartica'' has established itself in much of the lower 48 states.<ref name="Map"></ref> It has significant impact as an invasive species due to its allelopathic compounds, as discussed. In addition to this, buckthorn has an advantage when it comes phenology: extended leaf phenology. Buckthorn tends to flush out much earlier than native plants in its introduced range, allowing it to have photosynthetic advantage and to shade out native understory plants in the process, encouraging the establishment of a monoculture.<ref name="Knight"></ref> Additionally, the drupes on the shrub remain long past other native berries, providing a singular food source for birds which disperse the seeds as a result.<ref name="Knight"></ref> | Since its introduction to the United States in the 1800s, ''R. cathartica'' has established itself in much of the lower 48 states.<ref name="Map"></ref> It has significant impact as an invasive species due to its allelopathic compounds, as discussed. In addition to this, buckthorn has an advantage when it comes phenology: extended leaf phenology. Buckthorn tends to flush out much earlier than native plants in its introduced range, allowing it to have photosynthetic advantage and to shade out native understory plants in the process, encouraging the establishment of a monoculture.<ref name="Knight"></ref> Additionally, the drupes on the shrub remain long past other native berries, providing a singular food source for birds which disperse the seeds as a result.<ref name="Knight"></ref> | ||
| Line 52: | Line 52: | ||
== References == | == References == | ||
[6] EDDMapS. 2025. Early Detection & Distribution Mapping System. The University of Georgia - Center for Invasive Species and Ecosystem Health. Available online at http://www.eddmaps.org/; last accessed April 17, 2025. | |||
Revision as of 15:59, 17 April 2025

| |
| Kingdom: | Animalia |
|---|---|
| Phylum: | Platyhelminthes |
| Class: | Turbellaria |
| Order: | Tricladida |
| Family: | Geoplanidae |
| Genus: | Bipalium |
| Source: County of Brant [4] | |
Description
Bipalium, also known as the hammerhead worm, land planarian, or shovel-head garden worm, is a genus of invasive flatworm found in moist terrestrial environments in the northeastern United States [2]. The hammerhead worm is native to southeast Asia, and was introduced in 1891 [1]. This is easily identifiable by its distinct flattened body and oblong hammerhead. The worms are most often yellow, orange, or light brown, with a varying number of darker brown stripes running the length of the body [2]. Hammerhead worms pose a threat to natural ecosystems, as there are at least three species that have been found to feed exclusively on earthworms. They also pose a potential threat to agricultural operations, especially those operating in warm, moist environments [3].

Behaviors
Habitat and Range
R. cathartica contains a number of secondary compounds, present in both the fruit, leaves, root exudates, and other tissue of the plant[1] A specifically noted compound is that of emodin, which can impact germination of other plant seeds.[2] Emodin is present both in the root exudate of R. cathartica and in the fruit; due to the fact that the drupes often fall beneath the parent tree, both have a significant impact on germination. The presence of emodin also may contribute to the purgative effects of R. cathartica fruit, meaning that those unripe fruits which any animal may ingest will cause either regurgitation or very quick passing of the fruit.[2] This is significant as birds readily eat the fruit available.
Life Cycle
Impact as an Invasive Species
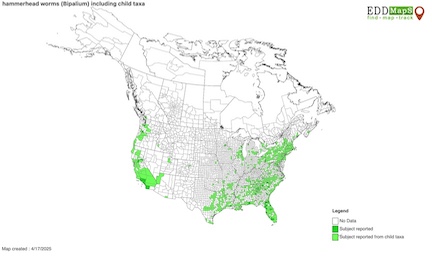
Since its introduction to the United States in the 1800s, R. cathartica has established itself in much of the lower 48 states.[3] It has significant impact as an invasive species due to its allelopathic compounds, as discussed. In addition to this, buckthorn has an advantage when it comes phenology: extended leaf phenology. Buckthorn tends to flush out much earlier than native plants in its introduced range, allowing it to have photosynthetic advantage and to shade out native understory plants in the process, encouraging the establishment of a monoculture.[2] Additionally, the drupes on the shrub remain long past other native berries, providing a singular food source for birds which disperse the seeds as a result.[2]
Another advantage is the preference that insects and mammalian herbivores have for native plants over R. cathartica in North America.[2] White-tailed deer, a significant herbivorous population, preferentially eat native plants due to the fact that R. cathartica causes them some illness, resulting in increased herbivorous pressure on those native plants. This also applies to small mammals which forage in the relevant environments. The presence of thick monospecific thickets which R. cathartica form tends to encourage small mammal foraging, due to an increase in available refuge; this limits the germination of native trees or other highly foraged plants.[4]
The result of the success of R. cathartica as a competitor means that when introduced in an area, buckthorn tends to form dense monospecific thickets which exclude any native competitors and often reducing understory plants to a minimum.[2] With monospecific thickets such as these, there is also the alteration of nutrient cycling, as the leaves of buckthorn are extremely high in Nitrogen. The change in litter types has significant impact on the soil ecology as it will rapidly increase high-N litter pools and then rapidly decrease them as longer lasting leaf litter is eradicated.[2] When this is combined with invasive earthworms such as L. terrestris the alteration of soil ecology in an area is notable, where quickly decomposing leaves and the high loss of detritus in the presence of invasive earthworms intersect and create rapidly cycling nutrient pools.
The allelopathic nature of R. cathartica also falls under the hypothesis of the novel weapons hypothesis; the hypothesis that non-native plants have competitive traits which native plants in the introduced range do not have a defense against.[5] The root exudates and secondary compounds of R. cathartica are hypothesized to inhibit not only the growth and germination of competitor plants, but also possibly the mutualist fungi which other plants form symbiosis with. As has been established, buckthorn contains emodin, a powerful allelochemical; in some cases this has been found to impact the mutualisms of other plants. The root exudates of R. cathartica were found to reduce arbuscular and vesicular colinization in Ulmus sp. despite both plants employing arbuscular mycorrhizal fungi in their mutualist relationships.[5] It is possible that there are other native competitors with fungal mutualisms which R. cathartica may indirectly degrade due to its allelopathy.
References
[6] EDDMapS. 2025. Early Detection & Distribution Mapping System. The University of Georgia - Center for Invasive Species and Ecosystem Health. Available online at http://www.eddmaps.org/; last accessed April 17, 2025.
- ↑ Cite error: Invalid
<ref>tag; no text was provided for refs namedSeltzner - ↑ 2.0 2.1 2.2 2.3 2.4 2.5 2.6 Cite error: Invalid
<ref>tag; no text was provided for refs namedKnight - ↑ Cite error: Invalid
<ref>tag; no text was provided for refs namedMap - ↑ Ryan M. Utz, Alysha Slater, Hannah R. Rosche, Walter P. Carson. "Do dense layers of invasive plants elevate the foragingintensity of small mammals in temperate deciduous forests? A case study from Pennsylvania, USA". NeoBiota 56: 73–88 (2020) doi: 10.3897/neobiota.56.4958. Retrieved 5/16/2022.
- ↑ 5.0 5.1 Pinzone, P., Potts, D., Pettibone, G. et al. "Do novel weapons that degrade mycorrhizal mutualisms promote species invasion?". Plant Ecol 219, 539–548 (2018). https://doi-org.gate.lib.buffalo.edu/10.1007/s11258-018-0816-4. Retrieved 5/16/2022.