Carbon-14: Difference between revisions
No edit summary |
No edit summary |
||
| Line 3: | Line 3: | ||
==''' Overview '''== | ==''' Overview '''== | ||
Carbon-14, also referred to as <sup>14</sup>C or radiocarbon, is one of the three naturally occurring isotopes of carbon in nature. <sup>14</sup>C is unstable, and has a half-life of 5700 +/- 30 years [1]. The other two forms of carbon are <sup>12</sup>C, which is what makes up 98% of all carbon in the atmosphere, and <sup>13</sup>C, which makes up around 1% of atmospheric carbon. Both <sup>12</sup>C and <sup>13</sup>C are stable isotopes, while <sup>14</sup>C is unstable. Within the context of [[ecology]], <sup>14</sup>C is used in two main processes: dating organic materials and tracing carbon pathways within ecosystems. | Carbon-14, also referred to as <sup>14</sup>C or radiocarbon, is one of the three naturally occurring isotopes of carbon in nature. <sup>14</sup>C is unstable, and has a half-life of 5700 +/- 30 years [1]. The other two forms of carbon are <sup>12</sup>C, which is what makes up 98% of all carbon in the atmosphere, and <sup>13</sup>C, which makes up around 1% of atmospheric carbon. Both <sup>12</sup>C and <sup>13</sup>C are stable isotopes, while <sup>14</sup>C is unstable. It only occurs in trace amounts naturally, around 1 <sup>14</sup>C atom for every 7.54 x 10<sup>11</sup> atoms of <sup>12</sup>C in the atmosphere. Within the context of [[ecology]], <sup>14</sup>C is used in two main processes: dating organic materials and tracing carbon pathways within ecosystems. | ||
== Uses == | == Uses == | ||
[[File:TreeDating.jpg|300px|thumb|left|Cross dating tree rings [2]]] | [[File:TreeDating.jpg|300px|thumb|left|Cross dating tree rings [2]]] | ||
===Tree Ring Dating=== | ===Tree Ring Dating=== | ||
<sup>14</sup>C is found | |||
===Carbon-14 Tracing=== | ===Carbon-14 Tracing=== | ||
Revision as of 14:43, 29 March 2025
Overview
Carbon-14, also referred to as 14C or radiocarbon, is one of the three naturally occurring isotopes of carbon in nature. 14C is unstable, and has a half-life of 5700 +/- 30 years [1]. The other two forms of carbon are 12C, which is what makes up 98% of all carbon in the atmosphere, and 13C, which makes up around 1% of atmospheric carbon. Both 12C and 13C are stable isotopes, while 14C is unstable. It only occurs in trace amounts naturally, around 1 14C atom for every 7.54 x 1011 atoms of 12C in the atmosphere. Within the context of ecology, 14C is used in two main processes: dating organic materials and tracing carbon pathways within ecosystems.
Uses
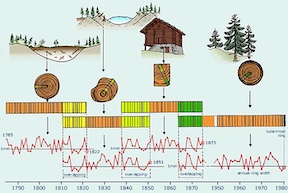
Tree Ring Dating
14C is found
Carbon-14 Tracing
EPS provides a stable interface for soil microbes to chemically work on attached substrate and suspended materials. In addition EPS can act as a store of carbon and other nutrients. The binding nature of EPS also helps reduce nutrient loss in soils from runoff.[1]
Analysis methods
Cation exchange resin (CER) extraction is currently considered the best method for accurately extracting EPS from soils.[2]This method shows the highest efficency with the least amount of modification to the original EPS composition when compared to other methods. This method works by replacing cations in the material being sampled. This destabilizes the structure and allows for separation through filtration and/or centrifuge.
References
- ↑ Lin, D., Ma, W., Jin, Z., Wang, Y., Huang, Q., Cai, P., 2016. Interactions of EPS with soil minerals: A combination study by ITC and CLSM. Colloids and Surfaces B: Biointerfaces 138, 10–16. https://doi.org/10.1016/j.colsurfb.2015.11.026
- ↑ Redmile-Gordon, M.A., Brookes, P.C., Evershed, R.P., Goulding, K.W.T., Hirsch, P.R., 2014. Measuring the soil-microbial interface: Extraction of extracellular polymeric substances (EPS) from soil biofilms. Soil Biology and Biochemistry 72, 163–171.