Methods for Sampling Macroarthropods: Difference between revisions
Created page with "Macroarthropods are a wildly diverse group, with several of their species scattered throughout the orders of Arthropoda. Most are large enough in size that they can be sampled..." |
No edit summary |
||
| Line 1: | Line 1: | ||
Macroarthropods are a wildly diverse group, with several of their species scattered throughout the orders of Arthropoda. Most are large enough in size that they can be sampled as individuals and counted with the naked eye, allowing for hand collection and sorting to be a viable means of population sampling.<sup>[1]</sup> | Macroarthropods are a wildly diverse group, with several of their species scattered throughout the orders of Arthropoda. Most are large enough in size that they can be sampled as individuals and counted with the naked eye, allowing for hand collection and sorting to be a viable means of population sampling.<sup>[1]</sup> | ||
[[File:Millipedelitter.png|thumb|right|Diplopoda]] | [[File:Millipedelitter.png|thumb|right|Diplopoda]] | ||
Many techniques rely on the natural characteristics of the specimen to aid in sampling of individuals. Cryptozoa, for instance, are crepuscular (active during twilight) or nocturnal and sampling techniques for these groupings rely on the use of light to drive individuals through the substrate | Many techniques rely on the natural characteristics of the specimen to aid in the sampling of individuals. Cryptozoa, for instance, are crepuscular (active during twilight) or nocturnal, and sampling techniques for these groupings rely on the use of light to drive individuals through the substrate toward a sampling mechanism.<sup>[2]</sup> Dependence on humidity levels or other defense mechanisms also drives many of the sampling techniques for this grouping of [[soil]] [[organisms]]. | ||
==Berlese or Tullgren Extraction== | ==Berlese or Tullgren Extraction== | ||
[[File:Tullgren.jpg|thumb|right|Berlese-Tullgren extraction funnel diagram]] | [[File:Tullgren.jpg|thumb|right|Berlese-Tullgren extraction funnel diagram]] | ||
A Berlese-Tullgren funnel, also known as a Berlese trap, utilizes a desiccation gradient to move | A Berlese-Tullgren funnel, also known as a Berlese trap, utilizes a desiccation gradient to move specimens through the substrate toward a collection vessel. Heat applied to the upper layers of a collected mass of substrate slowly dries it out from the top down, forcing the [[invertebrates]] to migrate down through the soil as they chase their desired levels of humidity. At the very bottom lies a collection vessel containing either a 70% alcohol solution or ethylene glycol which acts as a preservative.<sup>[2]</sup> | ||
===Sample Size=== | ===Sample Size=== | ||
Sample sizes for this technique can | Sample sizes for this technique can vary but are limited to a 0.1-2.5 m<sup>2</sup> collection.<sup>[1]</sup> Larger samples may be necessary for taxa that happen to have smaller population densities. This occurs if the population density is smaller than 1-2 individuals per mm<sup>2</sup>. In cases such as these, it may be necessary to conduct a thorough examination of the soil sample and hand-sort collected specimens. | ||
===DIY=== | ===DIY=== | ||
[[File:Tullgran funnels.jpg|thumb|left|Berlese-Tullgren funnel lab setup]] | [[File:Tullgran funnels.jpg|thumb|left|Berlese-Tullgren funnel lab setup]] | ||
Formerly, manufactured funnels are available for purchase however, these can be expensive with some units costing between $100 to $600 for a more complicated unit<sup>[2]</sup>. Many choose to construct their own versions using cheaper materials. Regardless of design, all have the same foundational structure. A heat lamp is placed over a funnel containing some sort of large diameter sieve to prevent debris from falling down the funnel opening. Below the opening of the funnel, a container filled with a preservative sits to collect specimens | Formerly, manufactured funnels are available for purchase however, these can be expensive with some units costing between $100 to $600 for a more complicated unit<sup>[2]</sup>. Many choose to construct their own versions using cheaper materials. Regardless of design, all have the same foundational structure. A heat lamp is placed over a funnel containing some sort of large-diameter sieve to prevent debris from falling down the funnel opening. Below the opening of the funnel, a container filled with a preservative sits to collect specimens that travel down through the substrate.<sup>[3]</sup> | ||
====''Materials Required'' <sup>[3]</sup>==== | ====''Materials Required'' <sup>[3]</sup>==== | ||
| Line 19: | Line 19: | ||
* Mesh screen (1/4" hardware cloth) | * Mesh screen (1/4" hardware cloth) | ||
* Ethanol (70-95%) or Isopropanol (70%) | * Ethanol (70-95%) or Isopropanol (70%) | ||
* Lamp with moveable neck (and incandescent bulb, for heat) | * Lamp with a moveable neck (and incandescent bulb, for heat) | ||
====''Assembly'' <sup>[3]</sup>==== | ====''Assembly'' <sup>[3]</sup>==== | ||
| Line 26: | Line 26: | ||
# Take the mesh screen and bend it so that it fits securely within inverted top of the 1-gallon jug. This will act as a stable platform for the sample. | # Take the mesh screen and bend it so that it fits securely within inverted top of the 1-gallon jug. This will act as a stable platform for the sample. | ||
# Place soil sample or leaf litter into funnel, resting it gently on top of mesh screen. | # Place soil sample or leaf litter into funnel, resting it gently on top of mesh screen. | ||
# Place lamp directly over soil sample. | # Place lamp directly over soil sample. The bulb should be approximately 20 cm away from the surface of the soil sample. Do not let the bulb touch the top of the soil itself. | ||
==Flotation== | ==Flotation== | ||
[[File:Extraction_flotation.png|thumb|right|Extraction of Collembola using flotation method]] | [[File:Extraction_flotation.png|thumb|right|Extraction of Collembola using flotation method]] | ||
Flotation methods are based on the principle that organic materials will have a density that is less than water allowing macroarthropod specimens to float to the top while minerals and soils will have a greater density, causing it to sink. Soil cores of 5-25mm in diameter and approximately 10-25 mm deep are taken as | Flotation methods are based on the principle that organic materials will have a density that is less than water allowing macroarthropod specimens to float to the top while minerals and soils will have a greater density, causing it to sink. Soil cores of 5-25mm in diameter and approximately 10-25 mm deep are taken as an indicator for macroarthropod population density.<sup>[1]</sup> Soil and solution are shaken together and allowed to settle. Organisms which float to the top of the water are then able to be collected and studied for population surveys.<sup>[4]</sup> | ||
Some studies have shown benefits | Some studies have shown the benefits of utilizing various hydrocarbon solvents such as gasoline as another layer of separation. Organisms were found to be caught in the gasoline layer, which floats on top of the water, while the soil, leaves and other bits of [[Organic Matter|organic matter]] from the sample were stuck in the water layer.<sup>[5]</sup> | ||
==Pitfall Trapping== | ==Pitfall Trapping== | ||
[[File:Pitfall.png|thumb|left|Diagram of a pitfall trap to collect ground dwelling insect]] | [[File:Pitfall.png|thumb|left|Diagram of a pitfall trap to collect ground dwelling insect]] | ||
While an inexpensive and rapid method for assessing community populations of macroarthropods, pitfall traps have limited capabilities when it comes to determining population size. Catches reflect both the density and mobility of [[arthropods]]. Ideal for species that spend most of their time on the ground, at its most basic form the pitfall trap consists of a container buried within the soil deep enough so that the top is flush with the ground’s surface. To prevent the organisms from escaping or eating other creatures that fall into the trap, a killing or preserving agent is placed | While an inexpensive and rapid method for assessing community populations of macroarthropods, pitfall traps have limited capabilities when it comes to determining population size. Catches reflect both the density and mobility of [[arthropods]]. Ideal for species that spend most of their time on the ground, at its most basic form the pitfall trap consists of a container buried within the soil deep enough so that the top is flush with the ground’s surface. To prevent the organisms from escaping or eating other creatures that fall into the trap, a killing or preserving agent is placed at the bottom. This is usually solutions such as soapy water or ethyl alcohol.<sup>[6]</sup> | ||
Revision as of 22:19, 22 April 2023
Macroarthropods are a wildly diverse group, with several of their species scattered throughout the orders of Arthropoda. Most are large enough in size that they can be sampled as individuals and counted with the naked eye, allowing for hand collection and sorting to be a viable means of population sampling.[1]

Many techniques rely on the natural characteristics of the specimen to aid in the sampling of individuals. Cryptozoa, for instance, are crepuscular (active during twilight) or nocturnal, and sampling techniques for these groupings rely on the use of light to drive individuals through the substrate toward a sampling mechanism.[2] Dependence on humidity levels or other defense mechanisms also drives many of the sampling techniques for this grouping of soil organisms.
Berlese or Tullgren Extraction
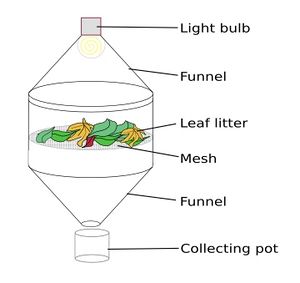
A Berlese-Tullgren funnel, also known as a Berlese trap, utilizes a desiccation gradient to move specimens through the substrate toward a collection vessel. Heat applied to the upper layers of a collected mass of substrate slowly dries it out from the top down, forcing the invertebrates to migrate down through the soil as they chase their desired levels of humidity. At the very bottom lies a collection vessel containing either a 70% alcohol solution or ethylene glycol which acts as a preservative.[2]
Sample Size
Sample sizes for this technique can vary but are limited to a 0.1-2.5 m2 collection.[1] Larger samples may be necessary for taxa that happen to have smaller population densities. This occurs if the population density is smaller than 1-2 individuals per mm2. In cases such as these, it may be necessary to conduct a thorough examination of the soil sample and hand-sort collected specimens.
DIY

Formerly, manufactured funnels are available for purchase however, these can be expensive with some units costing between $100 to $600 for a more complicated unit[2]. Many choose to construct their own versions using cheaper materials. Regardless of design, all have the same foundational structure. A heat lamp is placed over a funnel containing some sort of large-diameter sieve to prevent debris from falling down the funnel opening. Below the opening of the funnel, a container filled with a preservative sits to collect specimens that travel down through the substrate.[3]
Materials Required [3]
- 1-Gallon plastic jug (e.g., milk jug)
- Jar or container (e.g., 1-qt canning jar)
- Mesh screen (1/4" hardware cloth)
- Ethanol (70-95%) or Isopropanol (70%)
- Lamp with a moveable neck (and incandescent bulb, for heat)
Assembly [3]
- Cut the bottom off the 1-gallon jug. Only the top will be needed.
- Into the jar or container, pour a few centimeters of the ethanol or isopropanol.
- Take the mesh screen and bend it so that it fits securely within inverted top of the 1-gallon jug. This will act as a stable platform for the sample.
- Place soil sample or leaf litter into funnel, resting it gently on top of mesh screen.
- Place lamp directly over soil sample. The bulb should be approximately 20 cm away from the surface of the soil sample. Do not let the bulb touch the top of the soil itself.
Flotation

Flotation methods are based on the principle that organic materials will have a density that is less than water allowing macroarthropod specimens to float to the top while minerals and soils will have a greater density, causing it to sink. Soil cores of 5-25mm in diameter and approximately 10-25 mm deep are taken as an indicator for macroarthropod population density.[1] Soil and solution are shaken together and allowed to settle. Organisms which float to the top of the water are then able to be collected and studied for population surveys.[4]
Some studies have shown the benefits of utilizing various hydrocarbon solvents such as gasoline as another layer of separation. Organisms were found to be caught in the gasoline layer, which floats on top of the water, while the soil, leaves and other bits of organic matter from the sample were stuck in the water layer.[5]
Pitfall Trapping
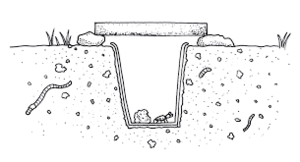
While an inexpensive and rapid method for assessing community populations of macroarthropods, pitfall traps have limited capabilities when it comes to determining population size. Catches reflect both the density and mobility of arthropods. Ideal for species that spend most of their time on the ground, at its most basic form the pitfall trap consists of a container buried within the soil deep enough so that the top is flush with the ground’s surface. To prevent the organisms from escaping or eating other creatures that fall into the trap, a killing or preserving agent is placed at the bottom. This is usually solutions such as soapy water or ethyl alcohol.[6]
References
[1] Coleman, D. C. 2004. Fundamentals of Soil Ecology. Elsevier Amstherdan.
[2] McWilliam, S. J. (n.d.). INVERTEBRATE CAPTURE TECHNIQUES. http://www.stevemcwilliam.co.uk/entomol/invcapt6.htm#:~:text=Tullgren%20funnels%20work%20extremely%20well,downwards%20to%20higher%20humidity%20levels.
[3] Constructing Berlese funnels to study invertebrate density and biodiversity. (n.d.). . https://www.carolina.com/teacher-resources/Interactive/constructing-berlese-funnels-study-invertebrate-density-biodiversity/tr19101.tr.
[4] Fowler, F., T. Wilcox, S. Orr, and W. Watson. 2020, November 1. Sampling efficacy and survival rates of labarrus pseudolividus (Coleoptera: Scarabaeidae) and onthophagus taurus (Coleoptera: Scarabaeidae) using flotation and Sieve-Separation Methodology. U.S. National Library of Medicine. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7751142/.
[5] Hale, W. G. 1964. A flotation method for extracting collembola from organic soils. The Journal of Animal Ecology 33:363.
[6] Virginia Tech. (n.d.). Using Pitfall Traps to Monitor Insect Activity. https://www.pubs.ext.vt.edu/content/dam/pubs_ext_vt_edu/444/444-041/444-041(ENTO-295P).pdf.