Phenazines: Difference between revisions
No edit summary |
No edit summary |
||
| (29 intermediate revisions by the same user not shown) | |||
| Line 3: | Line 3: | ||
==Biosynthesis and Chemistry== | ==Biosynthesis and Chemistry== | ||
[[File:PCA.png|right|thumb|caption|Figure 1. The chemical structure of phenazine-1-carboxylic acid (PCA). Notice the three carbon rings with the center ring containing two nitrogen atoms which is common to all phenazines.<ref name="(WITEGA)">WITEGA Laboratorien Berlin-Adlershof GmbH. (n.d.) Phenazine-1-carboxylic acid, [https://auftragssynthese.com/en/produkt/phenazine-1-carboxylic-acid-tubermycin-b/ https://auftragssynthese.com/en/produkt/phenazine-1-carboxylic-acid-tubermycin-b/]</ref>]] | |||
'''Redox-active metabolites (RAMs)''' can be generally defined as ‘natural byproducts’ or ‘secondary metabolites’ of soil bacteria.<ref name="(Dahlstrom et al., 2020)">Dahlstrom, Kurt; McRose, Darcy L.; Newman, Dianne K. (2020). Keystone metabolites of crop rhizosphere microbiomes. ''Current Biology''. Volume 30, Issue 19, Pages R1131-R1137, ISSN 0960-9822, [https://doi.org/10.1016/j.cub.2020.08.005 https://doi.org/10.1016/j.cub.2020.08.005]</ref> Although they are produced intracellularly and support cellular redox balancing, they are also secreted and may react extracellularly in the soil.<ref name="(Dahlstrom et al., 2020)"/> They accept and donate electrons to various soil constituents, hence why they are called ‘redox-active.'<ref name="(Dahlstrom et al., 2020)"/> | |||
[[File:Phenazine_Producer_Phylogeny.jpg|left|thumb|caption|Figure 2. The phylogenetic tree of the phenazine-producing bacteria with specific clades annotated around the tree<ref name="(Dar et al., 2020)"/>]] | |||
[[File:Phenazine_Colors.jpg|left|thumb|caption|Figure 3. a) Streak plate of ''P. aureofaciens'' which produces the bright orange phenazine 2-OHPCA. b) Various phenazines in test tubes displaying a spectrum of bright colors<ref name="(Price-Whelan et al., 2006)"/>]] | |||
Phenazines, in particular, are heterotricyclic nitrogen-containing metabolites.<ref name="(Mavrodi et al., 2010)">Mavrodi DV, Peever TL, Mavrodi OV, Parejko JA, Raaijmakers JM, Lemanceau P, Mazurier S, Heide L, Blankenfeldt W, Weller DM, Thomashow LS. (2010). [[Diversity]] and evolution of the phenazine biosynthesis pathway. ''Applied and Environmental Microbiology'' 76:866–879. [https://doi.org/10.1128/AEM.02009-09 https://doi.org/10.1128/AEM.02009-09]</ref> They are produced by ''Actinobacteria'' and ''Proteobacteria'', including the genera ''Pseudomonas spp.'', ''Streptomyces spp.'', and ''Pantoea agglomerans''.<ref name="(Dahlstrom et al., 2020)"/><ref name="(Pierson & Pierson, 2010)">Pierson, Leland S.; Pierson, Elizabeth A. (2010). "Metabolism and function of phenazines in bacteria: impacts on the behavior of bacteria in the environment and biotechnological processes". ''Applied Microbiology and Biotechnology''. 86 (6): 1659–1670. [https://link.springer.com/article/10.1007/s00253-010-2509-3 doi:10.1007/s00253-010-2509-3]</ref> All producer genomes contain the core biosynthesis genes ''phzA/BCDEFG'' that synthesize one of the two main phenazines which are precursors for all other derivatives: '''phenazine-1-carboxylic acid (PCA)''' (the chemical structure for which can be seen in Figure 1) in pseudomonads and '''phenazine-1,6-dicarboxylic acid (PDC)''' in most other species.<ref name="(Mavrodi et al., 2010)"/><ref name="(Dar et al., 2020)">Dar D, Thomashow LS, Weller DM, Newman DK. (2020). Global landscape of phenazine biosynthesis and biodegradation reveals species-specific colonization patterns in agricultural soils and crop microbiomes. ''Elife'' 9:e59726 [https://elifesciences.org/articles/59726 doi: 10.7554/eLife.59726]</ref> Auxiliary genes modify the core structure of these parent compounds to create different daughter phenazines with varying redox potentials and solubilities, ultimately affecting their beneficial function and toxicity.<ref name="(Laursen & Nielson, 2004)">Laursen JB, Nielsen J. (2004). Phenazine natural products: biosynthesis, synthetic analogues, and biological activity. ''Chemical Reviews'' 104:1663–1686. [https://doi.org/10.1021/cr020473j https://doi.org/10.1021/cr020473j]</ref> The variety of functional groups gives rise to a full spectrum of '''colors''' as well. For instance, 2-hydroxyphenazine-1-carboxylic acid (2-OHPCA) is bright orange, PCA is lemon yellow, 1-hydroxyphenazine (1-OHPHZ) is bright green, and 1-hydroxy-5-methylphenazine (pyocyanin, PYO) is bright blue as seen in Figure 3.<ref name="(Price-Whelan et al., 2006)">LPrice-Whelan, A., Dietrich, L. & Newman, D. Rethinking 'secondary' metabolism: physiological roles for phenazine antibiotics. ''Nat Chem Biol'' 2, 71–78 (2006). [https://doi.org/10.1038/nchembio764 https://doi.org/10.1038/nchembio764]</ref> | |||
The '''chemistry and toxicity''' of phenazines is dictated by the chemical microenvironment in the soil, namely oxygen, pH, and moisture.<ref name="(Dahlstrom et al., 2020)"/> For instance, at low pH, PCA is uncharged and more cell permeable, ultimately making it more toxic.<ref name="(Dahlstrom et al., 2020)"/> At high oxygen levels, PCA reacts with oxygen to create toxic reactive oxygen species (ROS).<ref name="(Dahlstrom et al., 2020)"/> In anoxic environments, PCA reacts with ferric iron minerals and produces ferrous iron through the process of '''reductive dissolution''' which may help solubilize mineral-adsorbed phosphorous.<ref name="(Dahlstrom et al., 2020)"/> | |||
==Effect on Competitive Fitness of Producer Organisms== | |||
The primary function of phenazines is for the '''competitive fitness''' of producer [[organisms]]. Phenazines allow their producers to survive under anoxic conditions, facilitate biofilm development, and aid acquisition of essential inorganic nutrients like iron and phosphorus.<ref name="(Dahlstrom et al., 2020)"/><ref name="(Dar et al., 2020)"/> Phenazine-producing strains have been shown to be better able to colonize the roots of wheat plants and persist in the rhizosphere than phenazine-lacking mutants.<ref name="(Mazzola et al., 1992)">Mazzola, M., Cook, R.J., Thomashow, L.S., Weller, D.M. & Pierson, L.S., III. Contribution of phenazine antibiotic biosynthesis to the ecological competence of fluorescent pseudomonads in soil habitats. ''Appl. Environ. Microbiol.'' 58, 2616–2624 (1992).</ref> | |||
In the same way that pseudomonads use phenazines to colonize [[plant roots]] in the rhizosphere, the opportunistic pathogen ''Pseudomonas aeruginosa'' uses the phenazine pyocyanin to colonize the biofilms of the lungs of cystic fibrosis patients.<ref name="(Villavicencio, 1998)">Villavicencio, R.T. The history of blue pus. J. ''Am. Coll. Surg.'' 187, 212–216 (1998).</ref> Pyocyanin has also been shown to facilitate interspecies antibiotic resistance, such as aiding the pathogenic fungi ''Candida albicans'' and ''Aspergillus fumigatus'' to colonize the cystic fibrosis lung.<ref name="(Morales et al., 2013)">D.K. Morales, N. Grahl, C. Okegbe, L.E.P. Dietrich, N.J. Jacobs, D.A. Hogan. Control of Candida albicans metabolism and biofilm formation by ''Pseudomonas aeruginosa'' phenazines. ''mBio'', 4 (2013), pp. e00526-00512</ref><ref name="(Briard et al., 2015)">B. Briard, P. Bomme, B.E. Lechner, G.L.A. Mislin, V. Lair, M.-C. Prévost, J.-P. Latgé, H. Haas, A. Beauvais. ''Pseudomonas aeruginosa'' manipulates redox and iron homeostasis of its microbiota partner ''Aspergillus fumigatus'' via phenazines. ''Sci. Rep.'', 5 (2015), p. 8220</ref> | |||
==Biocontrol of Beneficial and Pathogenic Fungi in Crop Soils== | |||
Phenazines can act as potent '''antibiotics''' by generating '''reactive oxygen species (ROS)'''. <ref name="(Baron et al., 1989)">Baron SS, Terranova G, Rowe JJ. (1989) Molecular mechanism of the antimicrobial action of pyocyanin. ''Current Microbiology'' 18:223–230. [https://doi.org/10.1007/BF01570296 https://doi.org/10.1007/BF01570296]</ref> These ROS interfere with the cellular respiration chains of competitor microbes, especially fungi.<ref name="(Baron et al., 1989)"/> Depending on the exposure, the ROS may not kill the fungi but just slow their growth.<ref name="(Dahlstrom et al., 2020)"/> In this way, the fungal organization of the [[rhizosphere]] may be partly determined by phenazines.<ref name="(Dahlstrom et al., 2020)"/> | |||
[[File:D._Japonica_in_maize_roots.png|right|thumb|caption|Figure 4. A maximal-projection cross-sectional image of a maize seedling root which shows that it has been colonized by the phenazine-producing bacteria ''D. japonica'' (in green).<ref name="(Dar et al., 2020)"/>]] | |||
The antibiotic nature of phenazines can be used to human advantage by suppressing '''pathogenic fungi''' from agricultural crops.<ref name="(Dahlstrom et al., 2020)"/> Phenazine-producing pseudomonads such as ''P. aureofaciens'', ''P. fluorescens'', and ''P. chlororaphis'' thrive as biofilms on the roots of plants and protect them from pathogenic fungi.<ref name="(Price-Whelan et al., 2006)"/> For instance, PAC can suppress ''Geaumannomyces graminis'' var. ''tritici'', the fungus that causes take-all disease in wheat.<ref name="(Thomashow et al., 1988)">L.S. Thomashow, D.M. Weller. Role of a phenazine antibiotic from ''Pseudomonas fluorescens'' in biological control of ''Gaeumannomyces graminis'' var. ''tritici''. ''J. Bacteriol''., 170 (1988), pp. 3499-3508.</ref> Another example is phenazine-1-carboxamide which can inhibit fungal pathogens like ''Fusarium oxysporum'' f. sp. ''radicis-lycopersici'', a cause of root rot in tomato plants.<ref name="(Bolwerk et al., 2003)">Bolwerk A, Lagopodi AL, Wijfjes AH, Lamers GE, Chin-A-Woeng TF, Lugtenberg BJ, Bloemberg GV. (2003) Interactions in the tomato rhizosphere of two ''Pseudomonas'' biocontrol strains with the phytopathogenic fungus ''Fusarium oxysporum'' f. sp. ''radicis-lycopersici''. ''Molecular Plant-Microbe Interactions'': MPMI 16:983–993. [https://doi.org/10.1094/MPMI.2003.16.11.983 https://doi.org/10.1094/MPMI.2003.16.11.983]</ref> Little is known about which bacterial clades and phenazine derivatives are naturally associated with various major crops and more research must be done to determine which bacteria can benefit which crops.<ref name="(Dar et al., 2020)"/> | |||
On the other hand, phenazines can act against '''beneficial fungi''', too. As soil becomes more arid due to '''climate change''', soil moisture will decrease and allow for higher oxygen penetration.<ref name="(Dahlstrom et al., 2020)"/> These conditions will facilitate an increase in the concentration of phenazine-producing bacteria and the phenazines themselves, while the phenazines will become more toxic from the creation of more ROS.<ref name="(Zeng & Dong, 2016)">Q. Zeng, Y. Dong, S. An. Bacterial community responses to soils along a latitudinal and vegetation gradient on the Loess Plateau, China. PLoS One, 11 (2016), p. e0152894</ref> Aridification (the less extreme version of [[desertification]]) will exclude beneficial fungi and decrease species diversity.<ref name="(Zeng & Dong, 2016)"/> | |||
Despite the stress that phenazines cause to fungi, they are often found living in proximity to phenazine-producers which suggests the presence of '''helper bacteria'''.<ref name="(Dahlstrom et al., 2020)"/> Certain bacteria possess redox-stress tolerance genes that protect them from fungally produced antibiotics, and it has been hypothesized that these same genes could help these bacteria tolerate the conditions caused by phenazine producers [1]. These helper bacteria may protect beneficial—or pathogenic—fungi from the redox-stress of phenazines.<ref name="(Dahlstrom et al., 2020)"/> Thus, the soil could be inoculated with these helper bacteria where phenazine concentrations are high to alleviate this stress.<ref name="(Dahlstrom et al., 2020)"/> | |||
Some redox-stress may increase plant health by suppressing fungal pathogens, while too much may negatively impact the fungal community by killing off beneficial fungi.<ref name="(Dahlstrom et al., 2020)"/> Therefore, a sustainable agricultural practice could be to irrigate for a short period of time when crops are young to inhibit phenazine producers. Later, as the producers build up anoxic microenvironments (biofilms), the phenazine producers may have a commensal or even mutualistic relationship with the crops.<ref name="(Dahlstrom et al., 2020)"/> | |||
Phenazines are found in relatively low concentrations in the soil. For instance, the upper extent of PCA concentrations in commercial wheat rhizospheres was found to be 1 μg PCA per gram fresh roots.<ref name="(Mavrodi et al., 2012)">Mavrodi DV, Mavrodi OV, Parejko JA, Bonsall RF, Kwak YS, Paulitz TC, Thomashow LS, Weller DM. (2012). Accumulation of the antibiotic phenazine-1-carboxylic acid in the rhizosphere of dryland cereals. ''Applied and Environmental Microbiology'' 78:804–812. [https://doi.org/10.1128/AEM.06784-11 https://doi.org/10.1128/AEM.06784-11]</ref> However, phenazines can be ‘re-reduced’ by microbes needing an electron acceptor in anoxic conditions which cycles the metabolites and allows the small concentration to be used many times over.<ref name="(Dahlstrom et al., 2020)"/> Since they are found in relatively low concentration in the soil but have such a pronounced impact on the microbial community in the rhizosphere, one researcher has dubbed them “'''keystone metabolites''',” a spin-off of the concept of a keystone species in macroecology.<ref name="(Dahlstrom et al., 2020)"/> | |||
==Promotion of Phosphorus Bioavailability for Sustainable Fertilizer== | |||
Phosphorus limits agricultural production due to a finite supply of phosphate rock and limited bioavailability from it adsorbing to iron minerals in the soil.<ref name="(Dahlstrom et al., 2020)"/> Consequently, farmers often overapply P fertilizers to compensate for the low bioavailability, leading to nutrient-rich run-off and [[eutrophication]].<ref name="(Dahlstrom et al., 2020)"/> Where phenazines can play an important role as sustainable fertilizer is as catalysts for phosphorus weathering by reducing iron minerals and liberating adsorbed phosphorus.<ref name="(Dahlstrom et al., 2020)"/> Scientists know that phenazines '''reduce Fe''' minerals, but they are not yet sure to what extent that they '''solubilize P'''.<ref name="(Stumm & Sulzberger, 1992)">W. Stumm, B. Sulzberger. The cycling of iron in natural environments: considerations based on laboratory studies of heterogenous redox processes. ''Geochimica et Cosmochimica Acta'', 56 (1992), pp. 3233-3257</ref> Bacteria have been shown to produce more phenazines under phosphorus-limited conditions, suggesting that the bacteria purposefully use phenazines for P acquisition.<ref name="(Dahlstrom et al., 2020)"/><ref name="(Cordell et al., 2009)">Cordell D, Drangert J-O, White S. (2009). The story of phosphorus: global food security and food for thought. ''Global Environmental Change'' 19:292–305. [https://doi.org/10.1016/j.gloenvcha.2008.10.009 https://doi.org/10.1016/j.gloenvcha.2008.10.009]</ref> | |||
==References== | ==References== | ||
<references /> | <references /> | ||
Latest revision as of 16:42, 4 May 2022
Phenazines are redox-active metabolites that are produced by certain soil bacteria and play an important role in the chemistry and ecology of the pedosphere. The compounds have been found across habitats and are especially abundant in root zones. Their redox activity makes them very reactive, and although they are found in relatively low concentrations, they have a disproportionate impact on the microbial ecology of the rhizosphere. Producer bacteria use phenazines to effectively compete with other soil microbes and acquire inorganic nutrients, which are processes that could be harnessed for sustainable agricultural practices in the future.
Biosynthesis and Chemistry

Redox-active metabolites (RAMs) can be generally defined as ‘natural byproducts’ or ‘secondary metabolites’ of soil bacteria.[2] Although they are produced intracellularly and support cellular redox balancing, they are also secreted and may react extracellularly in the soil.[2] They accept and donate electrons to various soil constituents, hence why they are called ‘redox-active.'[2]

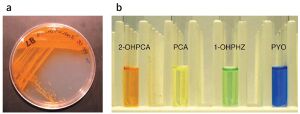
Phenazines, in particular, are heterotricyclic nitrogen-containing metabolites.[5] They are produced by Actinobacteria and Proteobacteria, including the genera Pseudomonas spp., Streptomyces spp., and Pantoea agglomerans.[2][6] All producer genomes contain the core biosynthesis genes phzA/BCDEFG that synthesize one of the two main phenazines which are precursors for all other derivatives: phenazine-1-carboxylic acid (PCA) (the chemical structure for which can be seen in Figure 1) in pseudomonads and phenazine-1,6-dicarboxylic acid (PDC) in most other species.[5][3] Auxiliary genes modify the core structure of these parent compounds to create different daughter phenazines with varying redox potentials and solubilities, ultimately affecting their beneficial function and toxicity.[7] The variety of functional groups gives rise to a full spectrum of colors as well. For instance, 2-hydroxyphenazine-1-carboxylic acid (2-OHPCA) is bright orange, PCA is lemon yellow, 1-hydroxyphenazine (1-OHPHZ) is bright green, and 1-hydroxy-5-methylphenazine (pyocyanin, PYO) is bright blue as seen in Figure 3.[4]
The chemistry and toxicity of phenazines is dictated by the chemical microenvironment in the soil, namely oxygen, pH, and moisture.[2] For instance, at low pH, PCA is uncharged and more cell permeable, ultimately making it more toxic.[2] At high oxygen levels, PCA reacts with oxygen to create toxic reactive oxygen species (ROS).[2] In anoxic environments, PCA reacts with ferric iron minerals and produces ferrous iron through the process of reductive dissolution which may help solubilize mineral-adsorbed phosphorous.[2]
Effect on Competitive Fitness of Producer Organisms
The primary function of phenazines is for the competitive fitness of producer organisms. Phenazines allow their producers to survive under anoxic conditions, facilitate biofilm development, and aid acquisition of essential inorganic nutrients like iron and phosphorus.[2][3] Phenazine-producing strains have been shown to be better able to colonize the roots of wheat plants and persist in the rhizosphere than phenazine-lacking mutants.[8]
In the same way that pseudomonads use phenazines to colonize plant roots in the rhizosphere, the opportunistic pathogen Pseudomonas aeruginosa uses the phenazine pyocyanin to colonize the biofilms of the lungs of cystic fibrosis patients.[9] Pyocyanin has also been shown to facilitate interspecies antibiotic resistance, such as aiding the pathogenic fungi Candida albicans and Aspergillus fumigatus to colonize the cystic fibrosis lung.[10][11]
Biocontrol of Beneficial and Pathogenic Fungi in Crop Soils
Phenazines can act as potent antibiotics by generating reactive oxygen species (ROS). [12] These ROS interfere with the cellular respiration chains of competitor microbes, especially fungi.[12] Depending on the exposure, the ROS may not kill the fungi but just slow their growth.[2] In this way, the fungal organization of the rhizosphere may be partly determined by phenazines.[2]

The antibiotic nature of phenazines can be used to human advantage by suppressing pathogenic fungi from agricultural crops.[2] Phenazine-producing pseudomonads such as P. aureofaciens, P. fluorescens, and P. chlororaphis thrive as biofilms on the roots of plants and protect them from pathogenic fungi.[4] For instance, PAC can suppress Geaumannomyces graminis var. tritici, the fungus that causes take-all disease in wheat.[13] Another example is phenazine-1-carboxamide which can inhibit fungal pathogens like Fusarium oxysporum f. sp. radicis-lycopersici, a cause of root rot in tomato plants.[14] Little is known about which bacterial clades and phenazine derivatives are naturally associated with various major crops and more research must be done to determine which bacteria can benefit which crops.[3]
On the other hand, phenazines can act against beneficial fungi, too. As soil becomes more arid due to climate change, soil moisture will decrease and allow for higher oxygen penetration.[2] These conditions will facilitate an increase in the concentration of phenazine-producing bacteria and the phenazines themselves, while the phenazines will become more toxic from the creation of more ROS.[15] Aridification (the less extreme version of desertification) will exclude beneficial fungi and decrease species diversity.[15]
Despite the stress that phenazines cause to fungi, they are often found living in proximity to phenazine-producers which suggests the presence of helper bacteria.[2] Certain bacteria possess redox-stress tolerance genes that protect them from fungally produced antibiotics, and it has been hypothesized that these same genes could help these bacteria tolerate the conditions caused by phenazine producers [1]. These helper bacteria may protect beneficial—or pathogenic—fungi from the redox-stress of phenazines.[2] Thus, the soil could be inoculated with these helper bacteria where phenazine concentrations are high to alleviate this stress.[2]
Some redox-stress may increase plant health by suppressing fungal pathogens, while too much may negatively impact the fungal community by killing off beneficial fungi.[2] Therefore, a sustainable agricultural practice could be to irrigate for a short period of time when crops are young to inhibit phenazine producers. Later, as the producers build up anoxic microenvironments (biofilms), the phenazine producers may have a commensal or even mutualistic relationship with the crops.[2]
Phenazines are found in relatively low concentrations in the soil. For instance, the upper extent of PCA concentrations in commercial wheat rhizospheres was found to be 1 μg PCA per gram fresh roots.[16] However, phenazines can be ‘re-reduced’ by microbes needing an electron acceptor in anoxic conditions which cycles the metabolites and allows the small concentration to be used many times over.[2] Since they are found in relatively low concentration in the soil but have such a pronounced impact on the microbial community in the rhizosphere, one researcher has dubbed them “keystone metabolites,” a spin-off of the concept of a keystone species in macroecology.[2]
Promotion of Phosphorus Bioavailability for Sustainable Fertilizer
Phosphorus limits agricultural production due to a finite supply of phosphate rock and limited bioavailability from it adsorbing to iron minerals in the soil.[2] Consequently, farmers often overapply P fertilizers to compensate for the low bioavailability, leading to nutrient-rich run-off and eutrophication.[2] Where phenazines can play an important role as sustainable fertilizer is as catalysts for phosphorus weathering by reducing iron minerals and liberating adsorbed phosphorus.[2] Scientists know that phenazines reduce Fe minerals, but they are not yet sure to what extent that they solubilize P.[17] Bacteria have been shown to produce more phenazines under phosphorus-limited conditions, suggesting that the bacteria purposefully use phenazines for P acquisition.[2][18]
References
- ↑ WITEGA Laboratorien Berlin-Adlershof GmbH. (n.d.) Phenazine-1-carboxylic acid, https://auftragssynthese.com/en/produkt/phenazine-1-carboxylic-acid-tubermycin-b/
- ↑ 2.00 2.01 2.02 2.03 2.04 2.05 2.06 2.07 2.08 2.09 2.10 2.11 2.12 2.13 2.14 2.15 2.16 2.17 2.18 2.19 2.20 2.21 2.22 2.23 Dahlstrom, Kurt; McRose, Darcy L.; Newman, Dianne K. (2020). Keystone metabolites of crop rhizosphere microbiomes. Current Biology. Volume 30, Issue 19, Pages R1131-R1137, ISSN 0960-9822, https://doi.org/10.1016/j.cub.2020.08.005
- ↑ 3.0 3.1 3.2 3.3 3.4 Dar D, Thomashow LS, Weller DM, Newman DK. (2020). Global landscape of phenazine biosynthesis and biodegradation reveals species-specific colonization patterns in agricultural soils and crop microbiomes. Elife 9:e59726 doi: 10.7554/eLife.59726
- ↑ 4.0 4.1 4.2 LPrice-Whelan, A., Dietrich, L. & Newman, D. Rethinking 'secondary' metabolism: physiological roles for phenazine antibiotics. Nat Chem Biol 2, 71–78 (2006). https://doi.org/10.1038/nchembio764
- ↑ 5.0 5.1 Mavrodi DV, Peever TL, Mavrodi OV, Parejko JA, Raaijmakers JM, Lemanceau P, Mazurier S, Heide L, Blankenfeldt W, Weller DM, Thomashow LS. (2010). Diversity and evolution of the phenazine biosynthesis pathway. Applied and Environmental Microbiology 76:866–879. https://doi.org/10.1128/AEM.02009-09
- ↑ Pierson, Leland S.; Pierson, Elizabeth A. (2010). "Metabolism and function of phenazines in bacteria: impacts on the behavior of bacteria in the environment and biotechnological processes". Applied Microbiology and Biotechnology. 86 (6): 1659–1670. doi:10.1007/s00253-010-2509-3
- ↑ Laursen JB, Nielsen J. (2004). Phenazine natural products: biosynthesis, synthetic analogues, and biological activity. Chemical Reviews 104:1663–1686. https://doi.org/10.1021/cr020473j
- ↑ Mazzola, M., Cook, R.J., Thomashow, L.S., Weller, D.M. & Pierson, L.S., III. Contribution of phenazine antibiotic biosynthesis to the ecological competence of fluorescent pseudomonads in soil habitats. Appl. Environ. Microbiol. 58, 2616–2624 (1992).
- ↑ Villavicencio, R.T. The history of blue pus. J. Am. Coll. Surg. 187, 212–216 (1998).
- ↑ D.K. Morales, N. Grahl, C. Okegbe, L.E.P. Dietrich, N.J. Jacobs, D.A. Hogan. Control of Candida albicans metabolism and biofilm formation by Pseudomonas aeruginosa phenazines. mBio, 4 (2013), pp. e00526-00512
- ↑ B. Briard, P. Bomme, B.E. Lechner, G.L.A. Mislin, V. Lair, M.-C. Prévost, J.-P. Latgé, H. Haas, A. Beauvais. Pseudomonas aeruginosa manipulates redox and iron homeostasis of its microbiota partner Aspergillus fumigatus via phenazines. Sci. Rep., 5 (2015), p. 8220
- ↑ 12.0 12.1 Baron SS, Terranova G, Rowe JJ. (1989) Molecular mechanism of the antimicrobial action of pyocyanin. Current Microbiology 18:223–230. https://doi.org/10.1007/BF01570296
- ↑ L.S. Thomashow, D.M. Weller. Role of a phenazine antibiotic from Pseudomonas fluorescens in biological control of Gaeumannomyces graminis var. tritici. J. Bacteriol., 170 (1988), pp. 3499-3508.
- ↑ Bolwerk A, Lagopodi AL, Wijfjes AH, Lamers GE, Chin-A-Woeng TF, Lugtenberg BJ, Bloemberg GV. (2003) Interactions in the tomato rhizosphere of two Pseudomonas biocontrol strains with the phytopathogenic fungus Fusarium oxysporum f. sp. radicis-lycopersici. Molecular Plant-Microbe Interactions: MPMI 16:983–993. https://doi.org/10.1094/MPMI.2003.16.11.983
- ↑ 15.0 15.1 Q. Zeng, Y. Dong, S. An. Bacterial community responses to soils along a latitudinal and vegetation gradient on the Loess Plateau, China. PLoS One, 11 (2016), p. e0152894
- ↑ Mavrodi DV, Mavrodi OV, Parejko JA, Bonsall RF, Kwak YS, Paulitz TC, Thomashow LS, Weller DM. (2012). Accumulation of the antibiotic phenazine-1-carboxylic acid in the rhizosphere of dryland cereals. Applied and Environmental Microbiology 78:804–812. https://doi.org/10.1128/AEM.06784-11
- ↑ W. Stumm, B. Sulzberger. The cycling of iron in natural environments: considerations based on laboratory studies of heterogenous redox processes. Geochimica et Cosmochimica Acta, 56 (1992), pp. 3233-3257
- ↑ Cordell D, Drangert J-O, White S. (2009). The story of phosphorus: global food security and food for thought. Global Environmental Change 19:292–305. https://doi.org/10.1016/j.gloenvcha.2008.10.009