Hydraulic Actions of Water: Difference between revisions
No edit summary |
No edit summary |
||
| Line 5: | Line 5: | ||
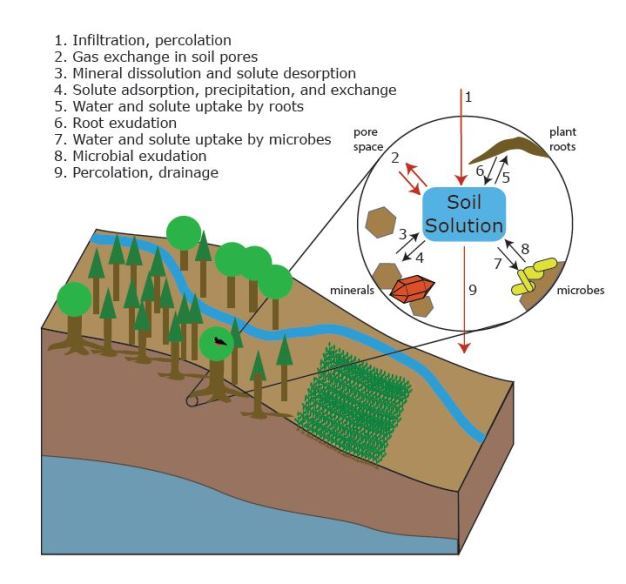
'''Figure 1:''' The interactions of water with solids, liquids, and gases [3] | |||
Water exists in three important phases: | Water exists in three important phases: | ||
Revision as of 20:52, 8 May 2018
Physical Properties of water
Water is present almost everywhere in soil held by strong capillary forces. In soils, water is a large contributor to biochemical factors [3]. Water influences the movement of solids, liquids, and gasses [3]. Water also is one of the greatest factors controlling flora growth and development [3].
Figure 1: The interactions of water with solids, liquids, and gases [3]
Water exists in three important phases:
Solid: Water freezes from the top down with the greatest density occurring at 4 degrees C. This is extremely important for biotic activity to exist below the freeze line [1]. The freezing of water contributes to Soil Processes through physical weathering of the parent material [1].
Liquid: Water has a high specific heat. Specific heat is the amount of energy required to change the temperature of a substance [4]. Through a high specific heat, water decreases rapid temperature fluctuations [1]. Liquid water is responsible for the movement of nutrients, runoff and pollutants through environments shaping the chemistry and physical properties of areas due to its solvency property. Liquid water is very important for plant growth through capillary action [4].
Vapor: Water vapor is a direct interaction between soil and the atmosphere. Dry soils will maintain a relativity humidity of 98% [2]. Soil organisms living in this humid environment rely on a habitat saturated with water through absorbing and loosing water via their integuments [1].
---
Molecular Properties of Water
Cohesion, molecular polarity, and hydrogen bonding all contribute to the movement of water in soil [2]
Hydrogen Bonding: The bonds between the positive hydrogen molecules and the negative oxygen molecules within water particles [2]
Cohesion:The attraction of water molecules between each other due to hydrogen bonding.
Adhesion: Also called “adsorption”, the attraction of water molecules to solid surfaces such as soil particles [2]. The surface area of particles determines the magnitude of adhesion [4]. Clay particles will have stronger adhesion forces compared to sand particles due to a larger surface area within the clay particles [4].
Capillary Forces: A combination of adhesion and surface tension [2]. Capillary rise is how tree roots retain groundwater, through a difference of pressures and the forces mentioned above. Surface tension refers to the attraction of water molecules to each other being greater than the attraction of the above air molecules to the water molecules [2].
Soil Water Types
Gravitational Water: Found in macropores within the soil, this form of water movement occurs when water moves rapidly through well drained soils [4]. This type of groundwater is considered not available to plants since it is temporally in place often draining away quickly [4]. Water can move through the unsaturated and saturated zones of soil via infiltration or percolation [2].
Capillary Water: This is the water available to plants [4]. Water molecules are held in soil through cohesion and adhesion forces being stronger than gravitational forces [4].
Hygroscopic Water: Water that forms a very thin film around soil particles not available to plants [3]. Clay particles hold onto this water strongly due to a large surface area [2]. Hygroscopic water is created through adhesion forces [4].
Soil Water measurements
Volumetric Water Content: Volume of water in a soil sample per unit of total soil volume [3].
Soil Wetness
Max Retentive Capacity: All pores are filled with water also referred to as saturation [2]. This occurs right after a rain event or snowmelt
Field Capacity: Maximum soil water content after gravity forces drain soil water [2]. This occurs around 1-3 days after a rain event and is used by plants [2].
Wilting Point: When the soil water content is at or below the level that plant roots can reach to absorb [2].
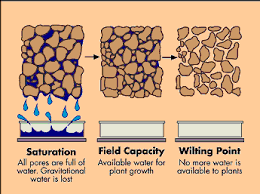
Figure 2: The Soil wetness parameters [5]
References:
[1] Coleman, D. C., D., A. C. J., & Hendrix, P. F. (2004). Fundamentals of soil ecology. Retrieved from https://ebookcentral.proquest.com
[2] “Lecture 2 : Soil Water: Characteristics and Behavior.” NPTEL, IIT Bombay, nptel.ac.in/courses/104103020/35.
[3] Duckworth, Owen W. “Soil Water: From Molecular Structure to Behavior.” Nature News, Nature Publishing Group, www.nature.com/scitable/knowledge/library/soil-water-from-molecular-structure-to-behavior-122155909.
[4] “Topic 9: Types of Soil Water.” Factors Affecting Plant Growth, broome.soil.ncsu.edu/ssc012/Lecture/topic9.htm.
[5] “Soil Science|Digital Textbook Library.” 2.1.1. Negative Impacts of Development, www.tankonyvtar.hu/en/tartalom/tamop425/0032_talajtan/ch07s02.html.