Carbon-14: Difference between revisions
No edit summary |
|||
| (15 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
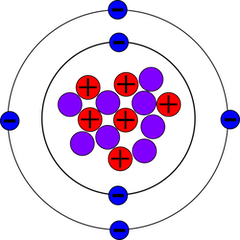
[[File:C-14.png|350px|thumb|right|Carbon-14 atom | [[File:C-14.png|350px|thumb|right|Carbon-14 atom containing 6 protons, 8 neutrons, and 6 electrons]] | ||
==''' Overview '''== | ==''' Overview '''== | ||
Carbon-14, also referred to as <sup>14</sup>C or radiocarbon, is one of the three naturally occurring isotopes of carbon in nature. <sup>14</sup>C is unstable, and has a half-life of 5700 +/- 30 years [1]. The other two forms of carbon are <sup>12</sup>C, which is what makes up 98% of all carbon in the atmosphere, and <sup>13</sup>C, which makes up around 1% of atmospheric carbon. Both <sup>12</sup>C and <sup>13</sup>C are stable isotopes, while <sup>14</sup>C is unstable. Within the context of [[ecology]], <sup>14</sup>C is used in two main processes: dating organic materials and tracing carbon pathways within ecosystems. | Carbon-14, also referred to as <sup>14</sup>C or radiocarbon, is one of the three naturally occurring isotopes of carbon in nature. <sup>14</sup>C is unstable, and has a half-life of 5700 +/- 30 years [1]. The other two forms of carbon are <sup>12</sup>C, which is what makes up 98% of all carbon in the atmosphere, and <sup>13</sup>C, which makes up around 1% of atmospheric carbon. Both <sup>12</sup>C and <sup>13</sup>C are stable isotopes, while <sup>14</sup>C is unstable. It only occurs in trace amounts naturally, around 1 <sup>14</sup>C atom for every 7.54 x 10<sup>11</sup> atoms of <sup>12</sup>C in the atmosphere. Within the context of [[ecology]], <sup>14</sup>C is used in two main processes: dating organic materials and tracing carbon pathways within ecosystems [2]. | ||
== Uses == | == Uses == | ||
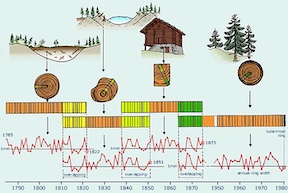
[[File: | [[File:TreeDating.jpg|300px|thumb|left|Cross dating tree rings [3]]] | ||
===Tree Ring Dating=== | ===Tree Ring Dating=== | ||
The | <sup>14</sup>C is found in the atmosphere, and although it occurs in trace amounts, it will be absorbed by trees like any other form of carbon. | ||
The science of dendochronology heavily relies on <sup>14</sup>C. Scientists are able to compare the tree rings of similar species growing in similar environments and match up their ages based on their rings [4]. From there, the <sup>14</sup>C content in each tree ring can be matched to the tree's age and year it was growing. Since the amount of <sup>14</sup>C in the atmosphere changes over time, scientists are also able to use the <sup>14</sup>C content in each tree ring to relate it to the atmospheric conditions at the time the tree was growing. Because of all of this data, it is possible to know the tree-ring chronologies for the past 12,400 years [5]. | |||
===Carbon-14 Tracing=== | ===Carbon-14 Tracing=== | ||
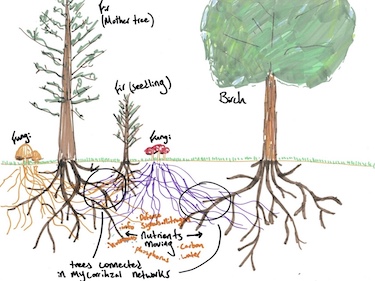
<sup>14</sup>C forms naturally in the atmosphere as a result of cosmic radiation. As it moves through the atmosphere, <sup>14</sup>C reacts with oxygen and forms CO<sub>2</sub>. That CO<sub>2</sub> will then be absorbed by trees to be converted into sugars that the tree uses for energy [6]. The <sup>14</sup>C becomes a part of the tree's tissue as it is absorbed. It may also be shunted to other trees via mycorrhizal fungi. In her book ''Finding The Mother Tree'', Suzanne Simard used this method to track the path of carbon through the trees' root systems, and prove that trees exchange <sup>14</sup>C through micorrhizal pathways [7]. This discovery of the vast networks of mycorrhizal has contributed greatly to forestry as a whole. | |||
[[File:HowTreesTalk.jpg|376px|thumb|right| | [[File:HowTreesTalk.jpg|376px|thumb|right|Suzanne Simard's drawing of carbon tracking through tree root systems [8]]] | ||
== | ==Analysis methods== | ||
The majority of <sup>14</sup>C analysis is used in determining ages of organic materials. <sup>14</sup>C can also tell information on [[soil]] carbon cycles find in soil trophic relationships. Analysis of <sup>14</sup>C in atmospheric and terrestrial environments is essential for understanding climate and soil environments. Through this analysis and the use of <sup>14</sup>C, we can predict possible future environmental impacts and global climate change in ecosystems [9]. | |||
== | ==References== | ||
<p>[1] Stark, A. M. (n.d.). Atmospheric carbon-14 measurements reveal natural production rate by cosmic rays | Lawrence Livermore | |||
National Laboratory. https://www.llnl.gov/article/42086/atmospheric-carbon-14-measurements-reveal-natural-production-rate-cosmic-rays. | |||
<p>[2] Cain, W. F., and H. E. Suess. 1976. Carbon 14 in tree rings. Journal of Geophysical Research (1896-1977) 81:3688–3694. | |||
<p> [3] NPR/TED, and 2020 10:13am. 1969, December 31. Suzanne Simard: How Do Trees Collaborate? https://www.northcountrypublicradio.org/news/npr/882828756/suzanne-simard-how-do-trees-collaborate. | |||
<p>[4] Douglass, A. E. 1941. Crossdating in Dendrochronology. Journal of Forestry. | |||
<p>[5] Guibal, F., and J. Guiot. 2021. Dendrochronology. Pages 117–122 Paleoclimatology. Springer, Cham. | |||
<p>[6] When trees “talk:” Researchers probe ancient wood for clues about massive solar storms | Arizona International. 2024, December 2. . https://international.arizona.edu/news/when-trees-talk-researchers-probe-ancient-wood-clues-about-massive-solar-storms. | |||
<p>[7] Simard, S. 2021, May 4. Finding the Mother Tree : Discovering the Wisdom of the Forest. https://web-p-ebscohost- | |||
com.gate.lib.buffalo.edu/ehost/ebookviewer/ebook?sid=3722a8f8-2b62-4b46-a151-54e5a387ffd8%40redis&vid=0&format=EK. | |||
<p>[8] Quaternary paleoenvironments - methods. (n.d.). . https://microsite.geo.uzh.ch/alpecole/static/course/lessons/28/28c.htm. | |||
<p>[9] Staddon, P. L. 2004. Carbon isotopes in functional [[Soil Ecology|soil ecology]]. Trends in Ecology & Evolution 19:148–154. | |||
Revision as of 13:27, 30 March 2025
Overview
Carbon-14, also referred to as 14C or radiocarbon, is one of the three naturally occurring isotopes of carbon in nature. 14C is unstable, and has a half-life of 5700 +/- 30 years [1]. The other two forms of carbon are 12C, which is what makes up 98% of all carbon in the atmosphere, and 13C, which makes up around 1% of atmospheric carbon. Both 12C and 13C are stable isotopes, while 14C is unstable. It only occurs in trace amounts naturally, around 1 14C atom for every 7.54 x 1011 atoms of 12C in the atmosphere. Within the context of ecology, 14C is used in two main processes: dating organic materials and tracing carbon pathways within ecosystems [2].
Uses
Tree Ring Dating
14C is found in the atmosphere, and although it occurs in trace amounts, it will be absorbed by trees like any other form of carbon. The science of dendochronology heavily relies on 14C. Scientists are able to compare the tree rings of similar species growing in similar environments and match up their ages based on their rings [4]. From there, the 14C content in each tree ring can be matched to the tree's age and year it was growing. Since the amount of 14C in the atmosphere changes over time, scientists are also able to use the 14C content in each tree ring to relate it to the atmospheric conditions at the time the tree was growing. Because of all of this data, it is possible to know the tree-ring chronologies for the past 12,400 years [5].
Carbon-14 Tracing
14C forms naturally in the atmosphere as a result of cosmic radiation. As it moves through the atmosphere, 14C reacts with oxygen and forms CO2. That CO2 will then be absorbed by trees to be converted into sugars that the tree uses for energy [6]. The 14C becomes a part of the tree's tissue as it is absorbed. It may also be shunted to other trees via mycorrhizal fungi. In her book Finding The Mother Tree, Suzanne Simard used this method to track the path of carbon through the trees' root systems, and prove that trees exchange 14C through micorrhizal pathways [7]. This discovery of the vast networks of mycorrhizal has contributed greatly to forestry as a whole.
Analysis methods
The majority of 14C analysis is used in determining ages of organic materials. 14C can also tell information on soil carbon cycles find in soil trophic relationships. Analysis of 14C in atmospheric and terrestrial environments is essential for understanding climate and soil environments. Through this analysis and the use of 14C, we can predict possible future environmental impacts and global climate change in ecosystems [9].
References
[1] Stark, A. M. (n.d.). Atmospheric carbon-14 measurements reveal natural production rate by cosmic rays | Lawrence Livermore National Laboratory. https://www.llnl.gov/article/42086/atmospheric-carbon-14-measurements-reveal-natural-production-rate-cosmic-rays.
[2] Cain, W. F., and H. E. Suess. 1976. Carbon 14 in tree rings. Journal of Geophysical Research (1896-1977) 81:3688–3694.
[3] NPR/TED, and 2020 10:13am. 1969, December 31. Suzanne Simard: How Do Trees Collaborate? https://www.northcountrypublicradio.org/news/npr/882828756/suzanne-simard-how-do-trees-collaborate.
[4] Douglass, A. E. 1941. Crossdating in Dendrochronology. Journal of Forestry.
[5] Guibal, F., and J. Guiot. 2021. Dendrochronology. Pages 117–122 Paleoclimatology. Springer, Cham.
[6] When trees “talk:” Researchers probe ancient wood for clues about massive solar storms | Arizona International. 2024, December 2. . https://international.arizona.edu/news/when-trees-talk-researchers-probe-ancient-wood-clues-about-massive-solar-storms.
[7] Simard, S. 2021, May 4. Finding the Mother Tree : Discovering the Wisdom of the Forest. https://web-p-ebscohost- com.gate.lib.buffalo.edu/ehost/ebookviewer/ebook?sid=3722a8f8-2b62-4b46-a151-54e5a387ffd8%40redis&vid=0&format=EK.
[8] Quaternary paleoenvironments - methods. (n.d.). . https://microsite.geo.uzh.ch/alpecole/static/course/lessons/28/28c.htm.
[9] Staddon, P. L. 2004. Carbon isotopes in functional soil ecology. Trends in Ecology & Evolution 19:148–154.